Applying Self-Assembly to Organocatalysis, Materials Science, and Medicinal Chemistry
Our research program revolves around the theme of synthetic macromolecular and supramolecular organic chemistry. We are particularly interested in the design and synthesis of functional macromolecules that fold into a well-defined secondary structure because these molecules have tremendous potential in the fields of medicinal chemistry, optoelectronics, and enzyme encapsulation, among many other applications in materials science and molecular recognition. Students involved in my research program will primarily learn techniques in synthetic organic chemistry; however, an appreciation for peptide synthesis and characterization, and supramolecular synthesis will also be developed.
The research goals of my group can be broadly classified into two categories: (a) developing molecules with well-defined conformational properties and (b) utilizing these materials in applications for which conformational order is crucial for function. Specifically, the following projects are underway in my laboratory.
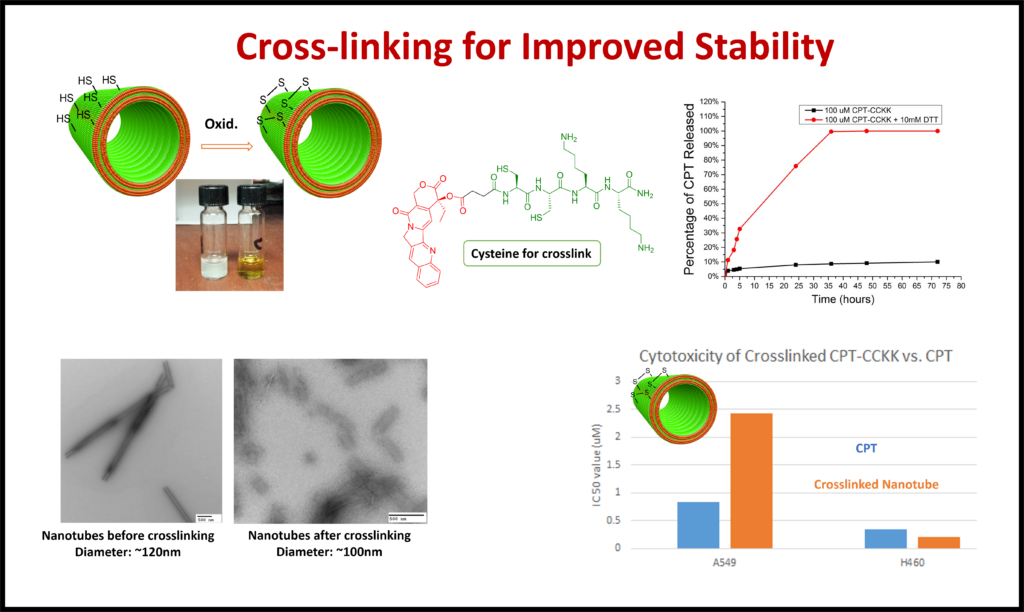
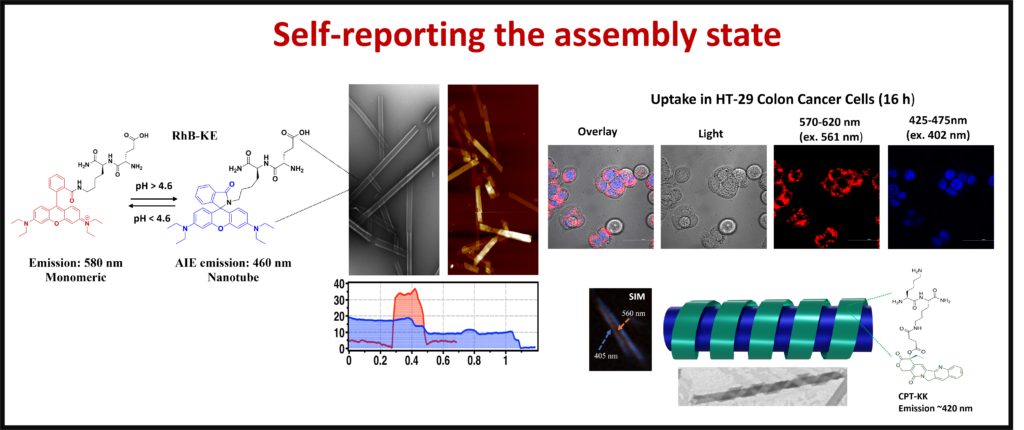
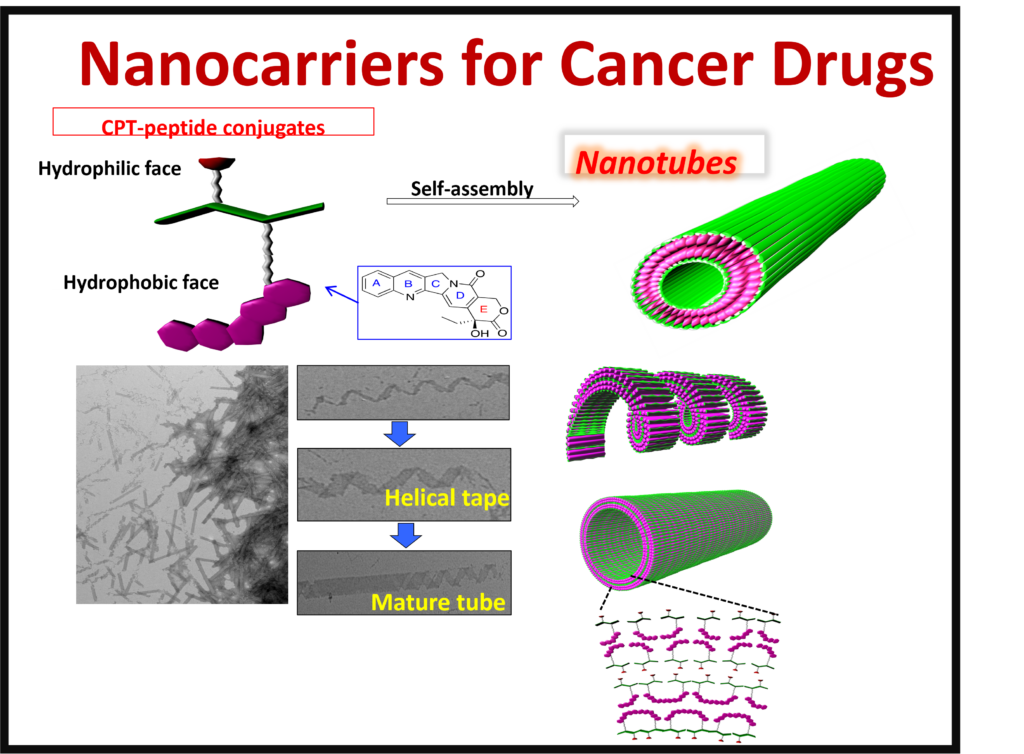
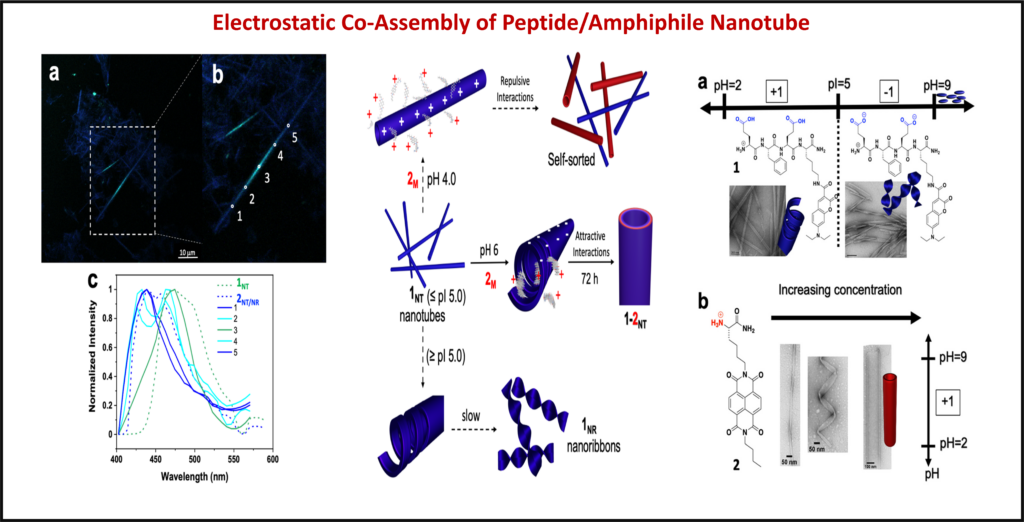
Medicinal Chemistry: There are many medicinal applications for self-assembled structures. Functionalizing short chain peptides has allowed us to create nanostructures with the potential to treat diseases such as diabetes and cancer. The nanostructures allow for control in the targeting and release of treatments.
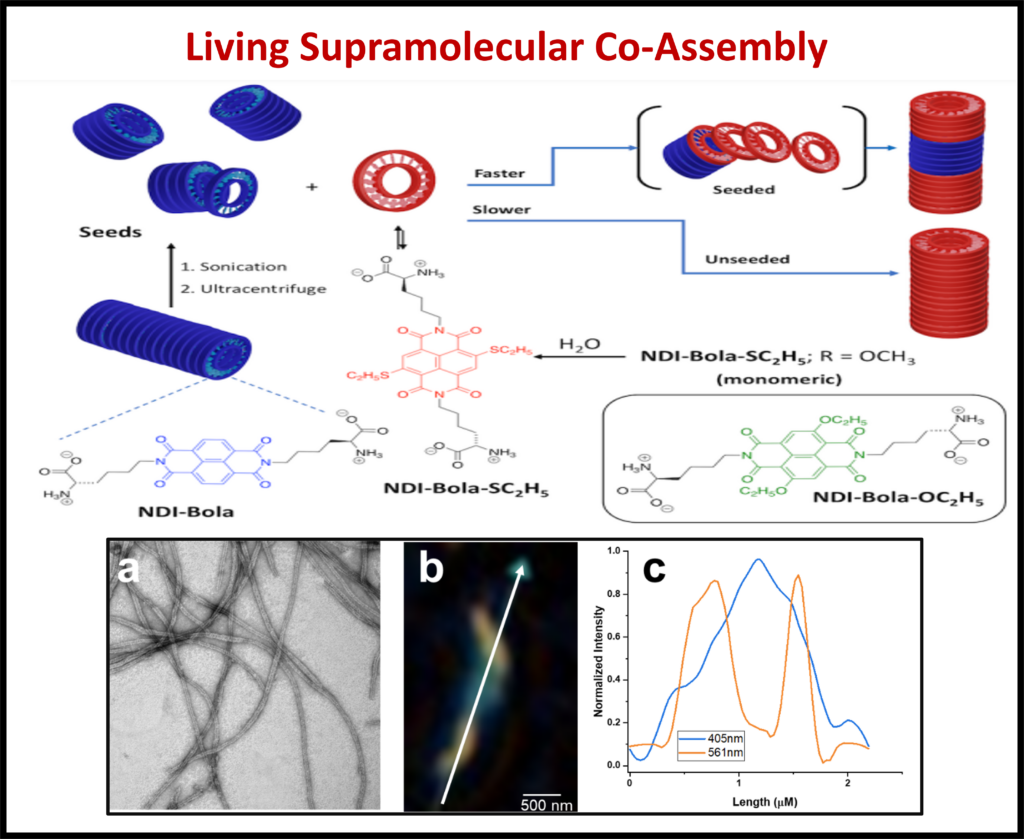
Functional Dissipative Systems: A select few dynamic nanostructures contain reversibly reactive compounds that respond to various stimuli to change their structure. By incorporating these structures into peptide sequences, we can give these dynamic properties to nanotubes and hydrogels allowing materials to change their physical characteristics.
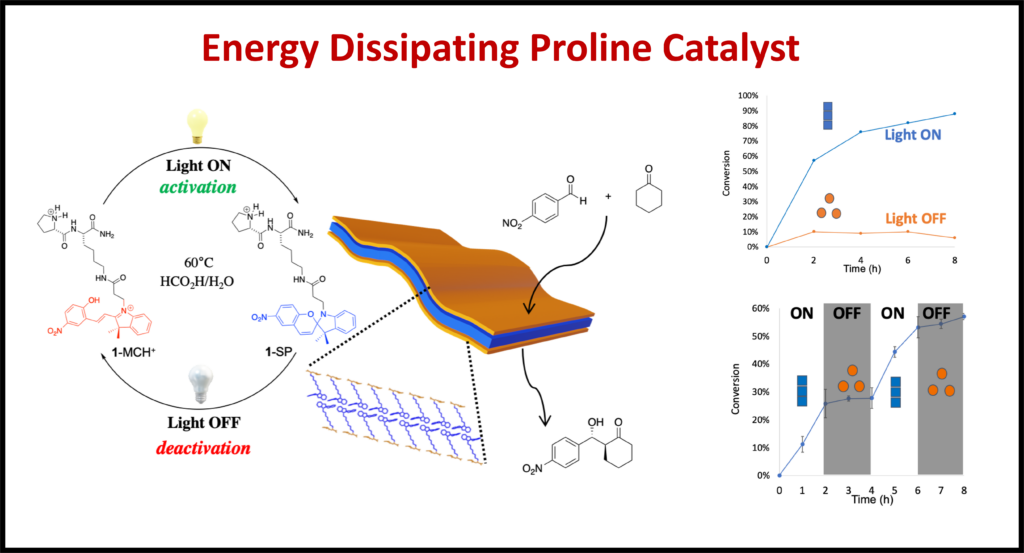
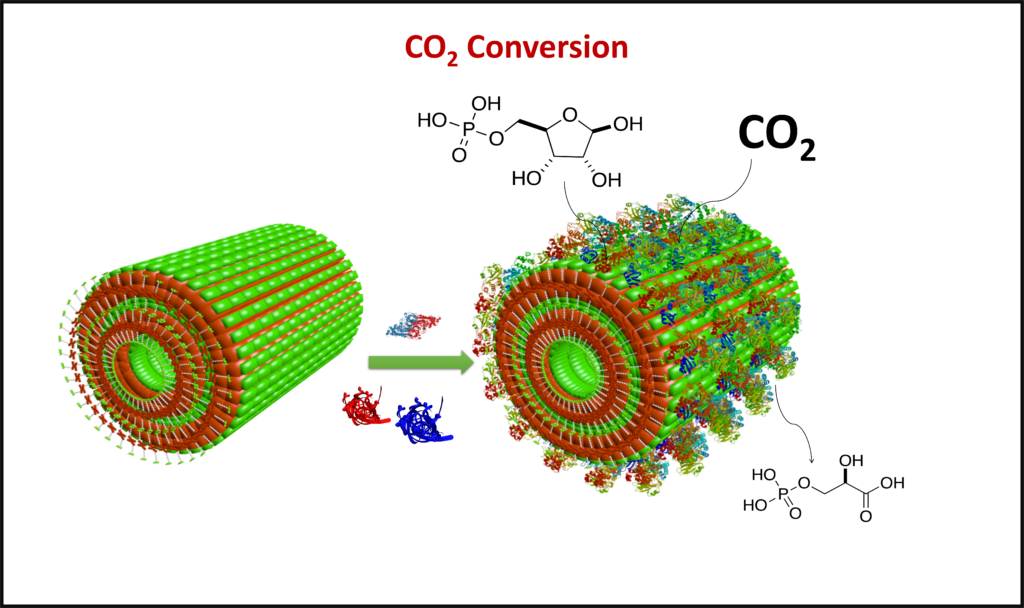
Enzyme Encapsulation: There have been many recent developments in the use of nanostructures to encapsulate enzymes. We attempt to use our structures to not only encapsulate the enzymes, but to also increase their activity for applications such as carbon capture.
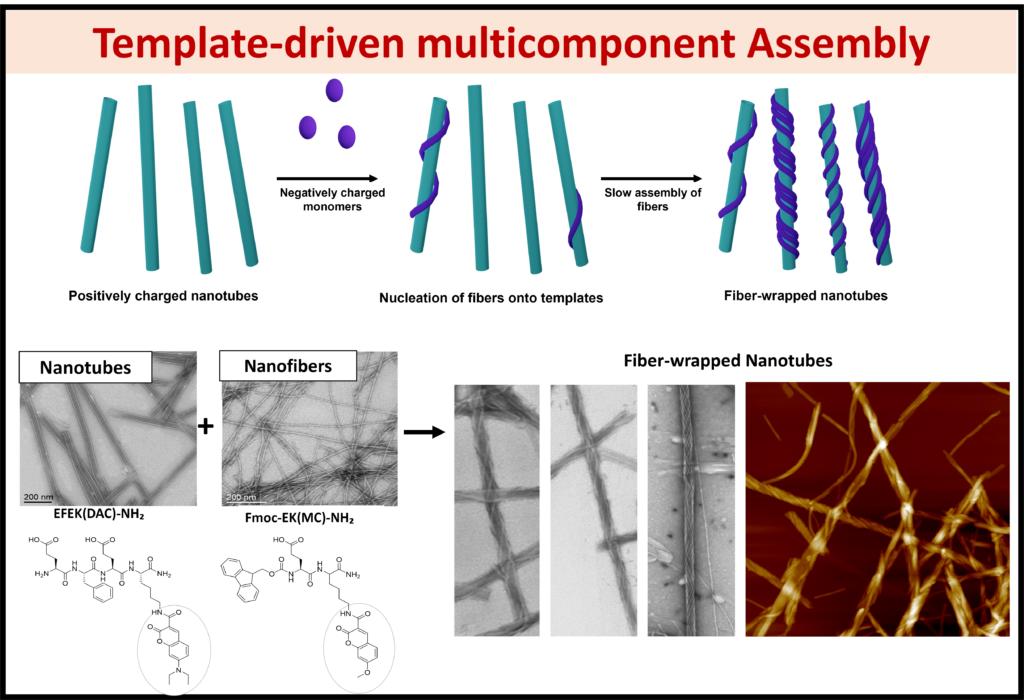
Co-Assembly: Using techniques like polymer wrapping and co-assembly, the functionalization of nanostructures allows for new and interesting advancements in the field of optoelectronics.