| Current Research |
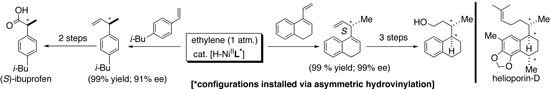
| We are engaged in two major areas of research that deals with new methodology for stereoselective synthesis: (a) Enantioselective catalysis of C-C, C-H and C-O bond formations. (b) Novel multicomponent cyclization methods that lead to vinyl Si, Sn and B derivatives, which are amenable to further stereoselective transformations. We are also pursuing applications of the newly developed methods for the synthesis of biologically relevant molecules. In the asymmetric catalysis area, we recently developed practical methods for C-C bond-formations based on hydrovinylation of vinylarenes, 1,3-dienes and strained olefins. Applications of this chemistry include a new synthesis of (S)-ibuprofen and a new approach to controlling the exocyclic side-chain stereochemistry in molecules such as helioporin D.
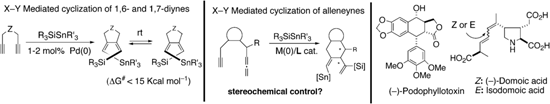
Examples of multicomponent addition/cyclization of diynes (giving novel axially chiral 1,3-dienes) and of allenynes, and potential targets that can be approached by this chemistry are shown below. In published work we have shown that related cyclization of allene-aldehydes allowed expeditious entry into highly alkylated indolizidines such as IND-223A.
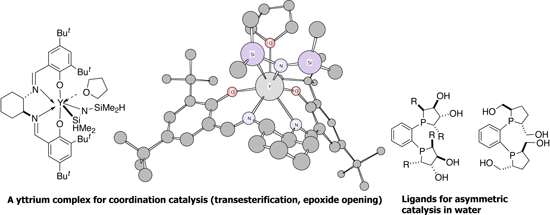
Other areas of our research interests are asymmetric hydrocyanation, coordination catalysis (kinetic resolution via epoxide opening, transesterification) by yttrium, new radical cyclization methods and development of organometallic reagents for aqueous organic chemistry.
|
The RajanBabu Group