Research
Reactivity of Dicopper Site in Nature
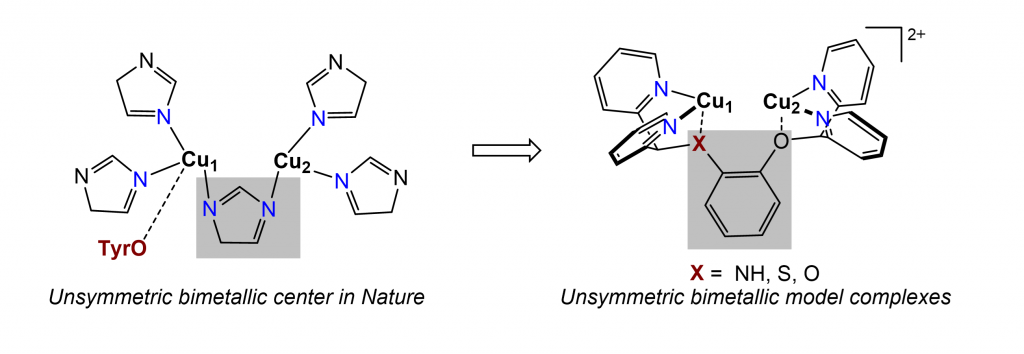
Metalloenzymes play a crucial role in multi-electron transfer reactions that are fundamental to all domains of life. These challenging cellular reactions, such as hydrocarbon oxidation and carbon dioxide reduction, are driven by the cooperative reactivity of bimetallic protein sites composed of Earth-abundant transition metals. The Zhang group aims to synthetically model biological centers with high reactivities that have yet to be replicated by synthetic systems. We aim to (1) understand the fundamental bioinorganic chemistry underlying the bimetallic synergy, (2) mimic the enzymatic reactivities with synthetic bimetallic catalysts, and (3) develop new reactions that are beyond the scope of what biology accomplishes itself.
Sustainable Organic Electrode Materials
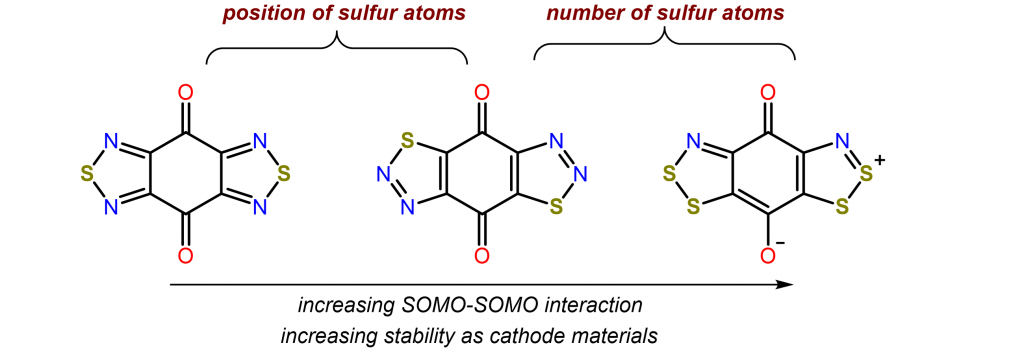
Conversion and storage of renewable energy to electrical power are key challenges across the world for realizing net zero carbon emission. On a smaller scale, further breakthroughs of secondary batteries are required to fulfill the future desires for electric vehicles (EV). With the driving range of commercial EV now approaches 300 miles, the slow charging rate of the current lithium-ion battery technology has become one of the most pressing issues that the electric vehicle industry faces today. The Zhang lab seeks to develop a general approach to prepare high rate organic radical battery. Our goal is to synthesize redox-active molecules with multiple reversible redox couples and systematically optimize their performance in batteries.
C-H Functionalization
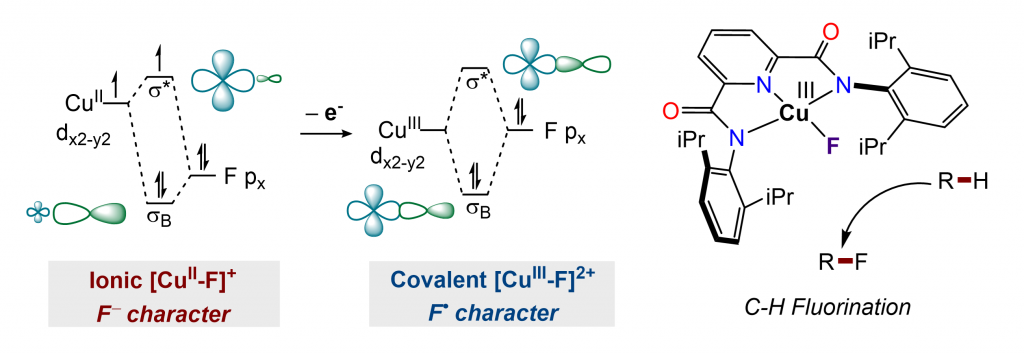
Despite the growing interest in the synthesis of fluorinated organic compounds, few methods are able to incorporate fluoride ion directly into alkyl C−H bonds. We are developing C(sp3)−H fluorination methods with formally copper(III) fluoride complexes. Quantum chemical calculations reveal significant fluorine radical character for copper(III) fluoride, suggesting their ability to initiate and terminate a C(sp3)-H fluorination sequence. The capability of copper(III) fluoride to perform both hydrogen atom abstraction and radical capture was leveraged to enable fluorination of allylic and benzylic C−H bonds and α-C−H bonds of ethers at room temperature.
Synthetic Tricopper Cluster
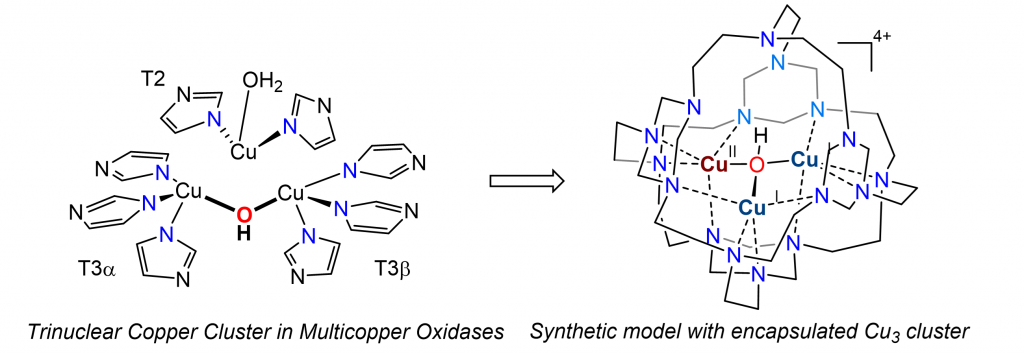
In nature, enzymes exploit locally structured functional groups to construct well-defined cavities near the metal active sites. These cavities provide reaction microenvironments that recognize substrates, activate substrates and minimize undesirable side reactions by protecting the reactive intermediates in inert confinement, thereby allowing incompatible reactions to proceed in parallel. We are interested in designing synthetic models that not only mimic the tricopper center in multicopper oxidases but also the unique cavity near the active site. Our studies show that tricopper hydroxo cluster in confinement exhibits redox behaviors distinct from those exposed to solvent.