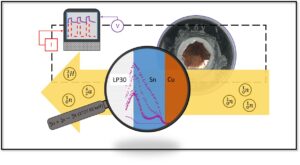
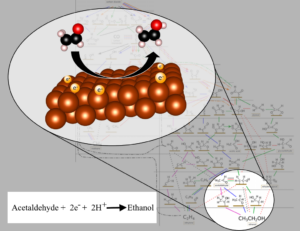
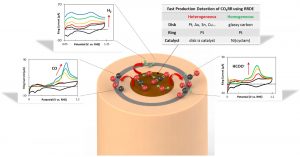
Summer 2024 Group Picture Pictured from left to right: Dr. José L. Lorié López, Dr. Zhihao Cui, Ariel Mendoza, Dr. Sung Gu Cho, Modeste Tegomoh, Scott Mitchell, Jiseon Hwang, Kassidy Aztergo, Chris Mangold, Dr. Anne Co, Dave Wood, Dr. Kug-Seung Lee, Maddie Palardy, Albert Wang, and Samantha Mendez
The primary focus of our research effort is in the fundamental studies of electrochemical reactions, electrocatalyst function and the design of new materials for furthering electrochemical technologies. Our lab is multidisciplinary, combining electrochemical, analytical, materials and physical chemistry techniques.
Main Research Goals
- Gain deeper fundamental understanding of electrocatalyst reactivity and selectivity
- Design, characterize, and evaluate new energy conversion and storage electrode materials
- Develop systematic methods of studying the mechanistic pathways of electrochemical reactions
- Utilize and develop in-situ methods for detecting reaction intermediates to infer reaction pathways