STRETCHING AND SORTING LIFE
Mechanotransduction – Cellular Adhesion – Structural Biology – Molecular Dynamics Simulations – Protein Biochemistry and Biophysics
 Living organisms rely on macroscopic and microscopic structures that produce and transform force to survive: from cell volume regulation to sound transduction and cellular adhesion, handling of forces is essential to life. Research in the Sotomayor group focuses on elucidating the molecular mechanisms underlying vertebrate mechanosensation and selective cellular adhesion. We use interdisciplinary and quantitative approaches to reveal structure-function relationships in macromolecular complexes such as those found in the mechanotransduction apparatus of inner-ear hair cells and in the cadherin adhesion machinery of epithelial tissues and synapses. These systems are ideal to study biological function at multiple levels: from single molecules to adhesion complexes and tissues, even up to animal behavior. Our combined experimental-computational research approach provides a unique structural framework to understand protein mechanics and function in hearing and balance, as well as in morphogenesis, cancer, and neuronal connectivity. It also provides the basis for engineering protein function in the context of mechanotransduction and cellular adhesion.
Living organisms rely on macroscopic and microscopic structures that produce and transform force to survive: from cell volume regulation to sound transduction and cellular adhesion, handling of forces is essential to life. Research in the Sotomayor group focuses on elucidating the molecular mechanisms underlying vertebrate mechanosensation and selective cellular adhesion. We use interdisciplinary and quantitative approaches to reveal structure-function relationships in macromolecular complexes such as those found in the mechanotransduction apparatus of inner-ear hair cells and in the cadherin adhesion machinery of epithelial tissues and synapses. These systems are ideal to study biological function at multiple levels: from single molecules to adhesion complexes and tissues, even up to animal behavior. Our combined experimental-computational research approach provides a unique structural framework to understand protein mechanics and function in hearing and balance, as well as in morphogenesis, cancer, and neuronal connectivity. It also provides the basis for engineering protein function in the context of mechanotransduction and cellular adhesion.
Ultimately, the success in understanding the mechanisms at play in mechanotransduction and adhesion will be measured by our ability to modify and manipulate these systems at will, for instance by creating stronger tip links, softer gating springs, transduction channels with reversed selectivity, cadherin bonds with designed specificity and mechanosensitivity, as well as photoswitchable adhesion molecules that can be used to turn on and off neuronal circuits or modify tissue development and mechanics.
CURRENT PROJECTS
We are involved in multiple projects and collaborations, including some focused on inner-ear proteins (cadherin-23, protocadherin-15, TMHS, TMIE, TMCs, CIBs, Ankyrin, Prestin), on cadherin complexes (adherens junctions, desmosomes, clustered protocadherins, delta protocadherins, cadherin-17, gut intermicrovillar cadherins), on ion channels (TRPN1 and TRPY1), on sonogenetics (collaboration with Chalasani group at Salk), and on enzymes that degrade plastic (PETases). Here are brief descriptions for some of these projects:
Structural Biology and Simulations of Proteins Involved in Hearing
Force Spectroscopy of Hair-Cell Tip Links
Molecular Connectomics: Binding Specificity in Novel Cadherin Complexes
RELATED LINKS
Structural Biology and Simulations of Proteins Involved in Hearing
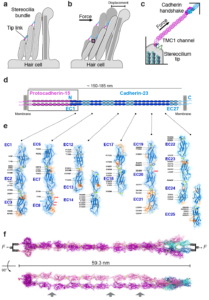
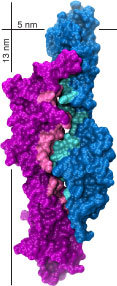
Mechanical forces produced by sound, gravity, and osmotic pressure are sensed by different organisms through multiple mechanisms that usually involve mechanosensitive ion channels and accessory proteins conveying tension. We are using a combination of X-ray crystallography, cryo-EM, and computational biology to determine the structure and dynamics of macromolecular complexes involved in vertebrate mechanotransduction. We are particularly interested in structures of cadherins (PCDH15 and CDH23) and membrane proteins and ion channels (TMIE, TMHS, and TMCs) involved in sound perception, as well as in testable predictions from large-scale all-atom molecular dynamics simulations of force transduction.
Movie – Tour of the hs PCDH15 EC1-MAD12 ex12a- + CDH23 EC1-2 model
Selected Related Publications
D. Choudhary*, Y. Narui*, B. L. Neel*, L. N. Wimalasena, C. F. Klanseck, P. De-la-Torre, C. Chen, R. Araya-Secchi, E. Tamilselvan, M. Sotomayor†. “Structural Determinants of Protocadherin-15 Mechanics and Function in Hearing and Balance Perception”. PNAS, 2020. Highlighted in: https://www.aps.anl.gov/APS-Science-Highlight/2020-10-12/two-molecular-handshakes-for-hearing
B. Pan*, N. Akyuz*, X-P. Liu*, Y. Asai, C. Nist-Lund, K. Kurima, B. H. Derfler, B. György, W. Limapichat, S. Walujkar, L. N. Wimalasena, M. Sotomayor, D. P. Corey†, and J. R. Holt†. “TMC1 Forms the Pore of Mechanosensory Transduction Channels in Vertebrate Inner Ear Hair Cells” Neuron, 99:736-753, 2018. Highlighted in: https://www.scientificamerican.com/article/a-40-year-quest-uncovers-hearings-holy-grail-protein/
A. Jaiganesh, P. De-la-Torre, A. A. Patel, D. J. Termine, F. Velez-Cortes, C. Chen, and M. Sotomayor†. “Zooming in on cadherin-23: Structural diversity and potential mechanisms of inherited deafness” Structure, 25:1210-1225, 2018.
Force Spectroscopy of Hair-Cell Tip Links
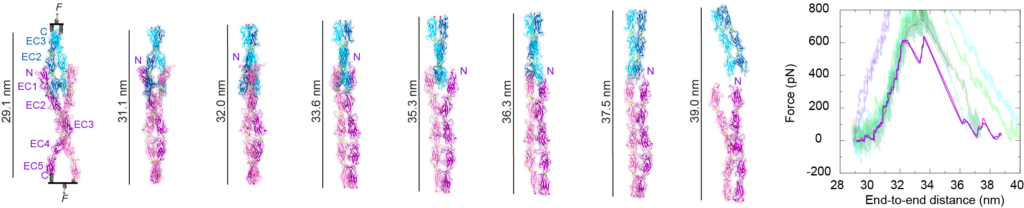
Hair cells are specialized and sensitive mechanoreceptors that transform mechanical stimuli into electrical signals in the inner ear. The tip link bond is essential for hair-cell mechanotransduction but little is known about the forces it can withstand and the molecular mechanisms underlying its noise-induced damage. The discovery of the tip-link molecular components and our resolution of the first X-ray crystallographic structure of part of the bond opened the door to explore the molecular determinants of its strength. We are using single-molecule force spectroscopy experiments and simulations to probe the strength of the tip-link bond and discover molecular mechanisms associated with its formation, evolution, rupture, and malfunction in deafness. The knowledge gained will allow us to design synthetic tip link bonds with enhanced mechanical strength, as well as molecular sensors to measure forces in vivo.
Movie – Forced Unbinding of Tip-Link Cadherins
Movie – Force Unfolding of Tip-Link Cadherins
Selected Related Publications
P. De-la-Torre, D. Choudhary, R. Araya-Secchi, Y. Narui, and M. Sotomayor†. “A Mechanically Weak Extracellular Membrane-Adjacent Domain Induces Dimerization of Protocadherin-15” Biophysical Journal, 115:2368-2385, 2018.
Y. Narui and M. Sotomayor†. “Tunning Inner-Ear Tip-Link Affinity Through Alternatively Spliced Variants of Protocadherin-15” Biochemistry, 57:1702-1710, 2018.
R. Araya-Secchi, B. L. Neel, and M. Sotomayor†. “An elastic element in the protocadherin-15 tip link of the inner ear” Nature Communications, 7:13458, 2016
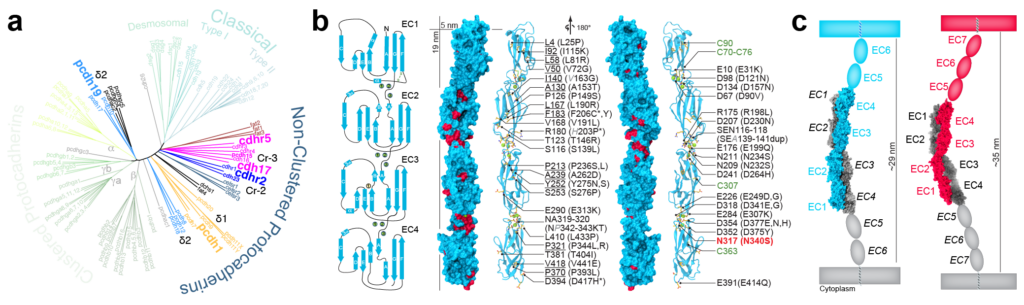
Molecular Connectomics: Binding Specificity in Novel Cadherin Complexes
Selective cell-cell adhesion is essential for tissue development and formation of complex and structured multicellular organs. Multiple families of cell-adhesion molecules have been identified; the cadherin superfamily of calcium-dependent adhesion proteins is one of the largest and has been implicated in tissue and organ morphogenesis and cancer. We are using bioinformatics and structural biology tools to discover novel cadherin complexes and to reveal the structural determinants of their binding specificity and biological function. The knowledge gained from the biophysical and biochemical characterization of these complexes will allow us to design synthetic cadherins with specific affinities and mechanical strength, as well as photoswitchable adhesion interfaces to control tissue development and neuronal connectivity.
Selected Related Publications
M. E. Gray*, Z. R. Johnson§*, D. Modak*, M. J. Tyska, M. Sotomayor†. “Species-Dependent Heterophilic and Homophilic Cadherin Interactions in Intestinal Intermicrovillar Links”. PLoS Biology 19(12):e3001463, 2021.
D. Modak and M. Sotomayor†. “Identification of an Adhesive Interface for the Non-Clustered δ1 Protocadherin-1 Involved in Respiratory Diseases”. Communications Biology, 2:354, 2019.
S. R. Cooper, J. D. Jontes†, and M. Sotomayor†. “Structural determinants of adhesion by Protocadherin-19 and implications for its role in epilepsy” eLife, 5:e18529, 2016.