RESEARCH HIGHLIGHTS
Two molecular handshakes for hearing
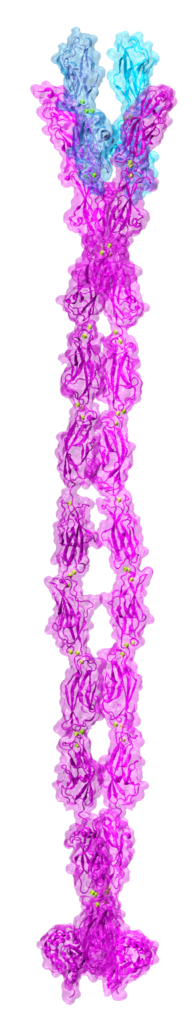 By the end of 2007 a consensus was emerging about which proteins formed the inner-ear hair-cell “tip link”, a fine filament essential for hearing and balance. Cadherin-23 was shown to form the upper part of tip links, while protocadherin-15 was found at the lower end–the two proteins interacting tip-to-tip to form an unprecedented cadherin heterotetramer. Yet, structural models of the complex were missing. We obtained X-ray crystal structures of cadherin-23 and protocadherin-15 fragments and of the interacting tips of both proteins in a “handshake” heterodimeric conformation (see “Cadherin Tip-Link Bond in HD” below). Using data from genetic studies we confirmed the physiological relevance of the handshake in vivo (see “Noddy mice: spinning around in a silent world” below). Yet from these studies it was still unclear how the heterotetramer would form, and the structure and elasticity for most of the tip-link proteins were still unknown. Almost a decade after our initial structural studies on tip links, we have solved significant parts of the puzzle: We revealed flexible domains in protocadherin-15 (see “A kinky protocadherin for hearing and balance” and “Structural diversity of protocadherin-15 linkers – Evolved weakness or hidden function?” below), discovered a new fold in a mechanically weak subdomain that induces parallel dimerization (see “Protocadherin-15’s Achilles’s heel” below), and finally solved the structure of the heterotetrameric complex showing two handshakes, with additional structures and simulations presenting the most complete atomistic model of the tip-link lower-end. A real “tour-de-force”, this work provided key insights into tip-link function and is guiding the design of mini-protocadherin-15 proteins to be used for gene therapies (see more in “Structural Determinants of Protocadherin-15 Mechanics and Function in Hearing and Balance Perception” PNAS, 117:24837-24848, 2020). Highlighted here.
By the end of 2007 a consensus was emerging about which proteins formed the inner-ear hair-cell “tip link”, a fine filament essential for hearing and balance. Cadherin-23 was shown to form the upper part of tip links, while protocadherin-15 was found at the lower end–the two proteins interacting tip-to-tip to form an unprecedented cadherin heterotetramer. Yet, structural models of the complex were missing. We obtained X-ray crystal structures of cadherin-23 and protocadherin-15 fragments and of the interacting tips of both proteins in a “handshake” heterodimeric conformation (see “Cadherin Tip-Link Bond in HD” below). Using data from genetic studies we confirmed the physiological relevance of the handshake in vivo (see “Noddy mice: spinning around in a silent world” below). Yet from these studies it was still unclear how the heterotetramer would form, and the structure and elasticity for most of the tip-link proteins were still unknown. Almost a decade after our initial structural studies on tip links, we have solved significant parts of the puzzle: We revealed flexible domains in protocadherin-15 (see “A kinky protocadherin for hearing and balance” and “Structural diversity of protocadherin-15 linkers – Evolved weakness or hidden function?” below), discovered a new fold in a mechanically weak subdomain that induces parallel dimerization (see “Protocadherin-15’s Achilles’s heel” below), and finally solved the structure of the heterotetrameric complex showing two handshakes, with additional structures and simulations presenting the most complete atomistic model of the tip-link lower-end. A real “tour-de-force”, this work provided key insights into tip-link function and is guiding the design of mini-protocadherin-15 proteins to be used for gene therapies (see more in “Structural Determinants of Protocadherin-15 Mechanics and Function in Hearing and Balance Perception” PNAS, 117:24837-24848, 2020). Highlighted here.
The first discovered protocadherin, PCDH1, comes into view
 Since the discovery of classical cadherins by Takeichi and Kemler in the 70s, multiple studies have thoroughly characterized the mechanisms by which classical cadherins use their extracellular domains with five extracellular cadherin (EC) repeats to glue cells together. As time passed, more cadherins were added to the superfamily, and it was clear that several members could not be classified as “classical” because their architecture, function, and binding mechanisms were distinct. Non-classical, non-clustered δ protocadherins form a unique set of subfamilies (δ1 and δ2) that are differentially expressed in the vertebrate brain and in various other organs. These protocadherins had been implicated in various diseases. However, when we started our research group, little was known about how δ protocadherins used their extracellular domains to mediate adhesion and about how their dysfunction caused disease. We focused first on PCDH19 a δ2 protocadherin with six EC repeats involved in epilepsy (see “A molecular forearm handshake to wire the brain” below). We followed up this work with studies of PCDH1, a δ1 protocadherin with seven EC repeats, ubiquitously expressed in various human tissues and involved in asthma and New World hantavirus infection. Our work revealed PCDH1’s molecular mechanism of adhesion and showed a first detailed view of how this and other proteins of the δ subfamilies may function. Furthermore, our structures showing PCDH1 EC1, to which the hantavirus glycoproteins bind, opened up avenues for drug discovery endeavors focused on preventing deadly hantavirus infections (see more in “Identification of an Adhesive Interface for the Non-Clustered δ1 Protocadherin-1 Involved in Respiratory Diseases”. Communications Biology, 2:354, 2019).
Since the discovery of classical cadherins by Takeichi and Kemler in the 70s, multiple studies have thoroughly characterized the mechanisms by which classical cadherins use their extracellular domains with five extracellular cadherin (EC) repeats to glue cells together. As time passed, more cadherins were added to the superfamily, and it was clear that several members could not be classified as “classical” because their architecture, function, and binding mechanisms were distinct. Non-classical, non-clustered δ protocadherins form a unique set of subfamilies (δ1 and δ2) that are differentially expressed in the vertebrate brain and in various other organs. These protocadherins had been implicated in various diseases. However, when we started our research group, little was known about how δ protocadherins used their extracellular domains to mediate adhesion and about how their dysfunction caused disease. We focused first on PCDH19 a δ2 protocadherin with six EC repeats involved in epilepsy (see “A molecular forearm handshake to wire the brain” below). We followed up this work with studies of PCDH1, a δ1 protocadherin with seven EC repeats, ubiquitously expressed in various human tissues and involved in asthma and New World hantavirus infection. Our work revealed PCDH1’s molecular mechanism of adhesion and showed a first detailed view of how this and other proteins of the δ subfamilies may function. Furthermore, our structures showing PCDH1 EC1, to which the hantavirus glycoproteins bind, opened up avenues for drug discovery endeavors focused on preventing deadly hantavirus infections (see more in “Identification of an Adhesive Interface for the Non-Clustered δ1 Protocadherin-1 Involved in Respiratory Diseases”. Communications Biology, 2:354, 2019).
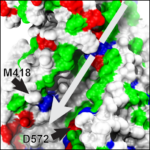
Protocadherin-15’s real Achilles heel
 Just as Achilles’ heel was left unprotected, evolution seems to have left a weak domain in various cadherin proteins, including those forming the tip link essential for hearing and balance. Our structure of a membrane adjacent domain (MAD12) in the tip-link protocadherin-15 protein revealed that this part of the protein does not fold as a typical extracellular cadherin (EC) repeat, but rather adopts a ferredoxin-like fold. Interestingly, our simulations predict that EC repeats are mechanically strong, while MAD12 might be mechanically weak. This is relevant because protocadherin-15 forms the lower end of the tip link filaments that pull on inner-ear transduction channels. The protocadherin-15 MAD12 also induces parallel dimerization, with the elastic response of the dimer involving unrolling and unfolding of MAD12s. Intriguingly, we predict that similar MADs are present in other cadherins, including the cadherin-23 protein that forms the upper part of the tip link (see more in “A Mechanically Weak Extracellular Membrane-Adjacent Domain Induces Dimerization of Protocadherin-15” Biophysical Journal, 115:2368-2385, 2018).
Just as Achilles’ heel was left unprotected, evolution seems to have left a weak domain in various cadherin proteins, including those forming the tip link essential for hearing and balance. Our structure of a membrane adjacent domain (MAD12) in the tip-link protocadherin-15 protein revealed that this part of the protein does not fold as a typical extracellular cadherin (EC) repeat, but rather adopts a ferredoxin-like fold. Interestingly, our simulations predict that EC repeats are mechanically strong, while MAD12 might be mechanically weak. This is relevant because protocadherin-15 forms the lower end of the tip link filaments that pull on inner-ear transduction channels. The protocadherin-15 MAD12 also induces parallel dimerization, with the elastic response of the dimer involving unrolling and unfolding of MAD12s. Intriguingly, we predict that similar MADs are present in other cadherins, including the cadherin-23 protein that forms the upper part of the tip link (see more in “A Mechanically Weak Extracellular Membrane-Adjacent Domain Induces Dimerization of Protocadherin-15” Biophysical Journal, 115:2368-2385, 2018).
A structural model of von Bekesy’s mechanical transformer for hearing
 “But since we have seen how, step by step, the anatomical structures in the ear localize the vibration forces in smaller and smaller compartments, it does not seem impossible that the final mechanical transformer is of molecular dimensions.” Georg von Bekesy, 1962. Almost sixty years after von Bekesy described the hair-cell “mechanical transformer”, we are still struggling to determine the molecular identity and architecture of the inner-ear mechanotransduction apparatus. However, genetic analyses and subsequent biophysical and biochemical studies have shown that at least TMC1 and three additional membrane proteins are part of the mechanotransduction complex. These proteins include protocadherin-15 (with its transmembrane domain), TMHS (aka LHFPL5), and TMIE. All of them are essential for hearing, yet which of these forms the pore of the transduction apparatus has been controversial. TMCs were predicted to have multiple transmembrane domains and to be distant homologs of TMEM16s membrane proteins, but decade-long efforts have failed to reveal their structure, a necessary step in the quest to elucidate the activation mechanisms and ion conduction pathway of the inner-ear mechanotransduction apparatus. Our collaborative effort with David P. Corey and Jeffrey Holt addressed this issue by building TMEM16-based homology models of TMC1 that were consistent with accessibility experiments that mapped the location of the hair-cell transduction channel pore. The experimentally validated homology models combined with our simulations predicted a pore that could permeate potassium ions and that, unlike other ion channels, was partially exposed to lipids. This was a landmark study providing the foundation for our current work on TMCs (see more in “TMC1 Forms the Pore of Mechanosensory Transduction Channels in Vertebrate Inner Ear Hair Cells” Neuron, 99:736-753, 2018). Highlighted here.
“But since we have seen how, step by step, the anatomical structures in the ear localize the vibration forces in smaller and smaller compartments, it does not seem impossible that the final mechanical transformer is of molecular dimensions.” Georg von Bekesy, 1962. Almost sixty years after von Bekesy described the hair-cell “mechanical transformer”, we are still struggling to determine the molecular identity and architecture of the inner-ear mechanotransduction apparatus. However, genetic analyses and subsequent biophysical and biochemical studies have shown that at least TMC1 and three additional membrane proteins are part of the mechanotransduction complex. These proteins include protocadherin-15 (with its transmembrane domain), TMHS (aka LHFPL5), and TMIE. All of them are essential for hearing, yet which of these forms the pore of the transduction apparatus has been controversial. TMCs were predicted to have multiple transmembrane domains and to be distant homologs of TMEM16s membrane proteins, but decade-long efforts have failed to reveal their structure, a necessary step in the quest to elucidate the activation mechanisms and ion conduction pathway of the inner-ear mechanotransduction apparatus. Our collaborative effort with David P. Corey and Jeffrey Holt addressed this issue by building TMEM16-based homology models of TMC1 that were consistent with accessibility experiments that mapped the location of the hair-cell transduction channel pore. The experimentally validated homology models combined with our simulations predicted a pore that could permeate potassium ions and that, unlike other ion channels, was partially exposed to lipids. This was a landmark study providing the foundation for our current work on TMCs (see more in “TMC1 Forms the Pore of Mechanosensory Transduction Channels in Vertebrate Inner Ear Hair Cells” Neuron, 99:736-753, 2018). Highlighted here.
Zooming in on cadherin-23, a protein involved in inherited deafness and blindness
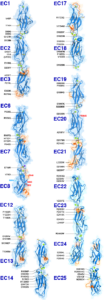 The first steps in sound and balance perception involve complex processes mediated by proteins forming the transduction apparatus of hair cells, the mechanoreceptors of the inner ear. Defects in these proteins are known to cause some forms of hereditary deafness and balance disorders. We used X-ray crystallography to obtain 13 structures that show in atomistic detail multiple parts of the extraordinarily large cadherin-23 protein, a key component of the inner-ear transduction apparatus. These structures show 18 out of 27 cadherin-23 extracellular cadherin (EC) repeats, providing a novel and unique view into 16 of these structurally diverse EC repeats and of 75 sites altered by deafness mutations. The structures include fragments EC1-3, EC6-8, EC12-14, and a large model of 9 EC repeats constructed from four overlapping structures of repeats EC17 to EC25. The structures revealed diverse features of cadherin-23’s architecture that determine how it functions in normal hearing, with additional analyses and experiments suggesting mechanisms by which cadherin-23 function is impaired in deafness. These findings have implications beyond hair-cell mechanotransduction, as cadherin-23 is also involved in inherited blindness and cancer (see more in “Zooming in on cadherin-23: Structural diversity and potential mechanisms of inherited deafness” Structure, 25:1210-1225, 2018).
The first steps in sound and balance perception involve complex processes mediated by proteins forming the transduction apparatus of hair cells, the mechanoreceptors of the inner ear. Defects in these proteins are known to cause some forms of hereditary deafness and balance disorders. We used X-ray crystallography to obtain 13 structures that show in atomistic detail multiple parts of the extraordinarily large cadherin-23 protein, a key component of the inner-ear transduction apparatus. These structures show 18 out of 27 cadherin-23 extracellular cadherin (EC) repeats, providing a novel and unique view into 16 of these structurally diverse EC repeats and of 75 sites altered by deafness mutations. The structures include fragments EC1-3, EC6-8, EC12-14, and a large model of 9 EC repeats constructed from four overlapping structures of repeats EC17 to EC25. The structures revealed diverse features of cadherin-23’s architecture that determine how it functions in normal hearing, with additional analyses and experiments suggesting mechanisms by which cadherin-23 function is impaired in deafness. These findings have implications beyond hair-cell mechanotransduction, as cadherin-23 is also involved in inherited blindness and cancer (see more in “Zooming in on cadherin-23: Structural diversity and potential mechanisms of inherited deafness” Structure, 25:1210-1225, 2018).
What makes a plastic-hungry enzyme more efficient at room temperature?
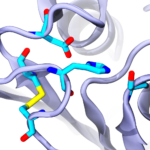 Ideonella sakaiensis bacteria were discovered near a Japanese recycling plant and were shown to be capable of breaking down the plastic polyethylene terephthalate (PET) to use it as a main source of carbon for cell growth. The secret weapon of these bacteria against PET seems to be a PET-degrading enzyme (PETase) that functions at room temperature more efficiently than other cutinases and carboxylesterases. To understand how PETase works, we solved its crystal structure and in collaboration with César Ramírez-Sarmiento and Loreto Parra did comparative molecular dynamics simulations of PETase and two other cutinases. Our experimental and computational results indicated that a key PETase disulfide bond, not present in other PET-degrading enzymes, helps in maintaining the structural integrity of an otherwise highly flexible active site. This enhanced flexibility may explain why PETase can degrade PET more efficiently than other cutinases at room temperature (see more in “Active Site Flexibility as a Hallmark for Efficient PET Degradation by I. sakaiensis PETase” Biophysical Journal, 114:1302-1312, 2018).
Ideonella sakaiensis bacteria were discovered near a Japanese recycling plant and were shown to be capable of breaking down the plastic polyethylene terephthalate (PET) to use it as a main source of carbon for cell growth. The secret weapon of these bacteria against PET seems to be a PET-degrading enzyme (PETase) that functions at room temperature more efficiently than other cutinases and carboxylesterases. To understand how PETase works, we solved its crystal structure and in collaboration with César Ramírez-Sarmiento and Loreto Parra did comparative molecular dynamics simulations of PETase and two other cutinases. Our experimental and computational results indicated that a key PETase disulfide bond, not present in other PET-degrading enzymes, helps in maintaining the structural integrity of an otherwise highly flexible active site. This enhanced flexibility may explain why PETase can degrade PET more efficiently than other cutinases at room temperature (see more in “Active Site Flexibility as a Hallmark for Efficient PET Degradation by I. sakaiensis PETase” Biophysical Journal, 114:1302-1312, 2018).
Hearing with protein handshakes of tunable strength
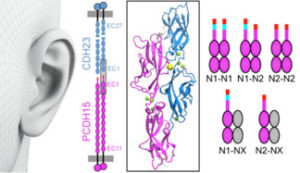 Our inner ears are equipped with sophisticated cellular machinery that can help us hear and process faint and loud sounds. This machinery relies on two proteins, cadherin-23 and protocadherin-15, that engage in a “handshake” interaction to form “tip links” essential for our senses of hearing and balance. Exposure to loud sounds can result in temporary rupture of this handshake, which may protect the transduction machinery from lasting damage. In contrast, inherited missense mutations can cause permanent disruption of the handshake bond and profound deafness. Intriguingly, the protocadherin-15 protein exists in different versions (splice isoforms) that have small and large modifications. We discovered, using X-ray crystallography, simulations, and binding assays, that some of these modifications alter the way in which protocadherin-15 interacts with cadherin-23, leading to strikingly weaker handshakes. Because the inner-ear tip links use two handshakes at a time, a combination of weak and strong bonds results in at least three types of tip links of varying strength. The next step is to determine which splice variants of protocadherin-15 are present in the inner ear, and what the biological relevance of such protein diversity might be. Perhaps tip links of different strength are optimized and used to hear specific sound frequencies (see more in “Tuning Inner-Ear Tip-Link Affinity Through Alternatively Spliced Variants of Protocadherin-15” Biochemistry, 57 (11), 1702-1710, 2018).
Our inner ears are equipped with sophisticated cellular machinery that can help us hear and process faint and loud sounds. This machinery relies on two proteins, cadherin-23 and protocadherin-15, that engage in a “handshake” interaction to form “tip links” essential for our senses of hearing and balance. Exposure to loud sounds can result in temporary rupture of this handshake, which may protect the transduction machinery from lasting damage. In contrast, inherited missense mutations can cause permanent disruption of the handshake bond and profound deafness. Intriguingly, the protocadherin-15 protein exists in different versions (splice isoforms) that have small and large modifications. We discovered, using X-ray crystallography, simulations, and binding assays, that some of these modifications alter the way in which protocadherin-15 interacts with cadherin-23, leading to strikingly weaker handshakes. Because the inner-ear tip links use two handshakes at a time, a combination of weak and strong bonds results in at least three types of tip links of varying strength. The next step is to determine which splice variants of protocadherin-15 are present in the inner ear, and what the biological relevance of such protein diversity might be. Perhaps tip links of different strength are optimized and used to hear specific sound frequencies (see more in “Tuning Inner-Ear Tip-Link Affinity Through Alternatively Spliced Variants of Protocadherin-15” Biochemistry, 57 (11), 1702-1710, 2018).
A fast method to detect good and bad inner-ear protein handshakes
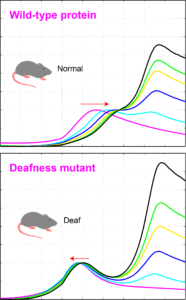 Proteins are complex biomolecular machines that play a plethora of vital roles in our body. They bind and interact with one another to form protein-protein complexes that are essential for multiple biological processes, such as cellular adhesion, processing of foreign substances that enter the body, and sensory perception. Small changes in protein structure (mutations) can impair or abolish binding to specific protein partners leading to deafness, epilepsy, and cancer. Thus, it is important to know how, and to what extent, proteins interact and bind with each other. There are multiple ways of measuring the extent of interaction (binding affinity) between two proteins, but most of them require sophisticated and expensive instrumentation, and often need large quantities of pure protein samples. We designed a high-throughput assay that allowed us to quickly test the interaction of two proteins using small amounts of samples. We used this assay to test the binding of protocadherin-15 to cadherin-23, two proteins that form a molecular handshake essential for hearing. We were able to detect proper handshake formation as an increase in the melting temperature of the complex, while failure to form the handshake due to mutations that cause deafness resulted in a clear decrease in the melting temperature of the protein mix. We also used this approach to find a mutation that enhanced binding affinity of the handshake complex by roughly two-fold that of the normal complex. We suggest that this methodology can be used to quickly compare binding affinities for some protein-protein complexes and to screen for drugs that can inhibit or enhance formation of protein complexes, perhaps to repair deficient interactions that lead to disease (see more in “Using thermal scanning assays to test protein-protein interactions of inner-ear cadherins” PLoS ONE, 12(12): e0189546, 2017).
Proteins are complex biomolecular machines that play a plethora of vital roles in our body. They bind and interact with one another to form protein-protein complexes that are essential for multiple biological processes, such as cellular adhesion, processing of foreign substances that enter the body, and sensory perception. Small changes in protein structure (mutations) can impair or abolish binding to specific protein partners leading to deafness, epilepsy, and cancer. Thus, it is important to know how, and to what extent, proteins interact and bind with each other. There are multiple ways of measuring the extent of interaction (binding affinity) between two proteins, but most of them require sophisticated and expensive instrumentation, and often need large quantities of pure protein samples. We designed a high-throughput assay that allowed us to quickly test the interaction of two proteins using small amounts of samples. We used this assay to test the binding of protocadherin-15 to cadherin-23, two proteins that form a molecular handshake essential for hearing. We were able to detect proper handshake formation as an increase in the melting temperature of the complex, while failure to form the handshake due to mutations that cause deafness resulted in a clear decrease in the melting temperature of the protein mix. We also used this approach to find a mutation that enhanced binding affinity of the handshake complex by roughly two-fold that of the normal complex. We suggest that this methodology can be used to quickly compare binding affinities for some protein-protein complexes and to screen for drugs that can inhibit or enhance formation of protein complexes, perhaps to repair deficient interactions that lead to disease (see more in “Using thermal scanning assays to test protein-protein interactions of inner-ear cadherins” PLoS ONE, 12(12): e0189546, 2017).
Structural diversity of protocadherin-15 linkers – Evolved weakness or hidden function?
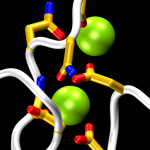 Cadherins form a large and diverse superfamily of proteins often involved in linking cells together. Critical to their function as “cellular glue” are their extracellular domains made of consecutive subunits called EC repeats. The EC repeats usually form rod-like hooks on the surface of cells that are strengthened and rigidified by calcium ions bound to linkers between repeats. In the absence of calcium, these linkers become flexible hinges and cadherin extracellular domains behave as weak and flexible ropes, unable to function and easy to break. The most conserved structural pieces among cadherins are the linkers between EC repeats, almost identical to each other and almost always hosting three calcium ions. Surprisingly, a member of the cadherin family essential for hearing (protocadherin-15) features linkers that are structurally diverse. Using sequence analyses and X-ray crystallography we found that the linker between repeats EC3 and EC4 sports only two calcium ions, rather than three, suggesting that this linker could be a weak, evolved “Achille’s heel” for protocadherin-15. However, molecular dynamics simulations show that the missing calcium binding site helps enhance the hinge flexibility of the EC3-4 linker without dramatically compromising its mechanical strength. These results hint at a hidden function for protocadherin-15, which is known to convey force to transduction channels of the inner ear to mediate sensory perception. Flexing of protocadherin-15 EC repeats, enhanced by its unusual linkers that bind only two calcium ions, might also help modulate ion channel gating by regulating the force required to open them (see more in “A Partial Calcium-Free Linker Confers Flexibility to Inner-Ear Protocadherin-15” Structure, 25:482-495, 2017).
Cadherins form a large and diverse superfamily of proteins often involved in linking cells together. Critical to their function as “cellular glue” are their extracellular domains made of consecutive subunits called EC repeats. The EC repeats usually form rod-like hooks on the surface of cells that are strengthened and rigidified by calcium ions bound to linkers between repeats. In the absence of calcium, these linkers become flexible hinges and cadherin extracellular domains behave as weak and flexible ropes, unable to function and easy to break. The most conserved structural pieces among cadherins are the linkers between EC repeats, almost identical to each other and almost always hosting three calcium ions. Surprisingly, a member of the cadherin family essential for hearing (protocadherin-15) features linkers that are structurally diverse. Using sequence analyses and X-ray crystallography we found that the linker between repeats EC3 and EC4 sports only two calcium ions, rather than three, suggesting that this linker could be a weak, evolved “Achille’s heel” for protocadherin-15. However, molecular dynamics simulations show that the missing calcium binding site helps enhance the hinge flexibility of the EC3-4 linker without dramatically compromising its mechanical strength. These results hint at a hidden function for protocadherin-15, which is known to convey force to transduction channels of the inner ear to mediate sensory perception. Flexing of protocadherin-15 EC repeats, enhanced by its unusual linkers that bind only two calcium ions, might also help modulate ion channel gating by regulating the force required to open them (see more in “A Partial Calcium-Free Linker Confers Flexibility to Inner-Ear Protocadherin-15” Structure, 25:482-495, 2017).
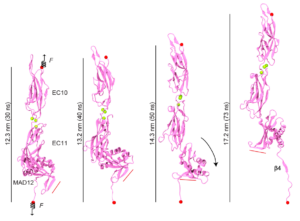
A kinky protocadherin for hearing and balance
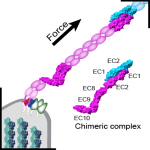 Deep in the inner ear, fine nanoscopic protein filaments called “tip links” are constantly subjected to forces induced by sound and head movements. As tip links are put under tension, they convey force to mechanosensitive channels that gate open to trigger minute ionic currents that initiate sensory perception. Tip links are essential for hearing and balance and are made of two enormous proteins with long extracellular domains that have multiple rigidifying calcium-binding sites. One of these proteins, called protocadherin-15, lacks some of these calcium-binding sites, yet the functional consequences of missing them were unknown. Using X-ray crystallography, we were able to determine the first high-resolution structure of the human version of a protocadherin-15 piece lacking these calcium-binding sites. This structure revealed a kinked conformation in the crystal (“in crystallo“), rather than the typical extended and rigid conformation observed for pieces that have calcium-binding sites. A second structure of the mouse version of this piece also showed the kinked conformation in crystallo, and small-angle X-ray scattering experiments that provide a low-resolution view of the protein shape confirmed the existence of this kink in solution. In addition, molecular dynamics simulations predicted that this kinked conformation is stable “in silico” (in the computer), and that it can withstand the resting tension expected for tip links in vivo, reversibly straightening only when forces from sound and head movements push tip links’ tension above a specific force threshold. Structures, experiments, and simulations thus predict that some parts of protocadherin-15 can behave as elastic springs that modulate the gating of inner-ear mechanosensitive channels. These results have implications for numerous other cadherins predicted to lack some calcium-binding sites and are providing a unique high-resolution molecular view of the initial steps that underlie inner-ear sensory perception (see more in “An elastic element in the protocadherin-15 tip link of the inner ear” Nature Communications, 7:13458, 2016).
Deep in the inner ear, fine nanoscopic protein filaments called “tip links” are constantly subjected to forces induced by sound and head movements. As tip links are put under tension, they convey force to mechanosensitive channels that gate open to trigger minute ionic currents that initiate sensory perception. Tip links are essential for hearing and balance and are made of two enormous proteins with long extracellular domains that have multiple rigidifying calcium-binding sites. One of these proteins, called protocadherin-15, lacks some of these calcium-binding sites, yet the functional consequences of missing them were unknown. Using X-ray crystallography, we were able to determine the first high-resolution structure of the human version of a protocadherin-15 piece lacking these calcium-binding sites. This structure revealed a kinked conformation in the crystal (“in crystallo“), rather than the typical extended and rigid conformation observed for pieces that have calcium-binding sites. A second structure of the mouse version of this piece also showed the kinked conformation in crystallo, and small-angle X-ray scattering experiments that provide a low-resolution view of the protein shape confirmed the existence of this kink in solution. In addition, molecular dynamics simulations predicted that this kinked conformation is stable “in silico” (in the computer), and that it can withstand the resting tension expected for tip links in vivo, reversibly straightening only when forces from sound and head movements push tip links’ tension above a specific force threshold. Structures, experiments, and simulations thus predict that some parts of protocadherin-15 can behave as elastic springs that modulate the gating of inner-ear mechanosensitive channels. These results have implications for numerous other cadherins predicted to lack some calcium-binding sites and are providing a unique high-resolution molecular view of the initial steps that underlie inner-ear sensory perception (see more in “An elastic element in the protocadherin-15 tip link of the inner ear” Nature Communications, 7:13458, 2016).
A molecular “forearm handshake” to wire the brain
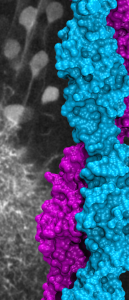 The basic building blocks of the brain are neuronal cells that during development need to connect with one another to form neural circuits. A small mistake leading to incorrect connections can result in brain abnormalities and other disorders. The glue that links neurons together is made of proteins present on the neuron’s surface. These proteins enable neuronal cells to stick to their correct partners like Velcro. One of these proteins is called Protocadherin-19 (PCDH19), which belongs to a family of proteins that localize to the surface of neuronal cells. When a PCDH19 protein molecule on one neuron finds another PCDH19 on an adjacent neuron, they bind to one another and link those neurons together. Similar adhesive bonds are formed by other members of this protein family, yet how PCDH19 forms the bond or how different proteins within this family are able to distinguish between one another is unclear. In collaboration with the Jontes lab, we used X-ray crystallography to visualize for the first time the PCDH19 structure and the adhesive bond it forms to link neuronal cells. It turns out that PCDH19 molecules from adjacent cells engage in a “forearm handshake” that other members of the family may use as well. The structure also allowed us to see many protein sites that are altered (mutated) in patients with a female-limited, infant-onset form of epilepsy (PCDH19-FE). Some of the PCDH19-FE mutations causing epilepsy occur at the binding interface of the forearm handshake, and experiments confirmed that these mutations impair the formation of the adhesive bond. This work revealed for the first time the binding mechanism used by PCDH19 to connect neuronal cells, the molecular basis of some PCDH19-FE mutations causing epilepsy, and the relevance of the “forearm handshake” for brain wiring in vivo (see more in “Structural determinants of adhesion by Protocadherin-19 and implications for its role in epilepsy” eLife, 5:e18529, 2016).
The basic building blocks of the brain are neuronal cells that during development need to connect with one another to form neural circuits. A small mistake leading to incorrect connections can result in brain abnormalities and other disorders. The glue that links neurons together is made of proteins present on the neuron’s surface. These proteins enable neuronal cells to stick to their correct partners like Velcro. One of these proteins is called Protocadherin-19 (PCDH19), which belongs to a family of proteins that localize to the surface of neuronal cells. When a PCDH19 protein molecule on one neuron finds another PCDH19 on an adjacent neuron, they bind to one another and link those neurons together. Similar adhesive bonds are formed by other members of this protein family, yet how PCDH19 forms the bond or how different proteins within this family are able to distinguish between one another is unclear. In collaboration with the Jontes lab, we used X-ray crystallography to visualize for the first time the PCDH19 structure and the adhesive bond it forms to link neuronal cells. It turns out that PCDH19 molecules from adjacent cells engage in a “forearm handshake” that other members of the family may use as well. The structure also allowed us to see many protein sites that are altered (mutated) in patients with a female-limited, infant-onset form of epilepsy (PCDH19-FE). Some of the PCDH19-FE mutations causing epilepsy occur at the binding interface of the forearm handshake, and experiments confirmed that these mutations impair the formation of the adhesive bond. This work revealed for the first time the binding mechanism used by PCDH19 to connect neuronal cells, the molecular basis of some PCDH19-FE mutations causing epilepsy, and the relevance of the “forearm handshake” for brain wiring in vivo (see more in “Structural determinants of adhesion by Protocadherin-19 and implications for its role in epilepsy” eLife, 5:e18529, 2016).
Noddy mice: spinning around in a silent world
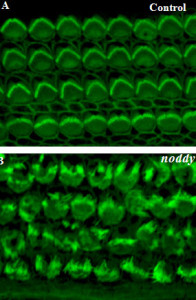 We use our inner ear in everyday life, all the time. Not only to hear the world around us, but also to detect motion of our head, to maintain our gaze, and to discern up from down. Could you imagine going around the world without the senses of hearing and balance? That is exactly what happens to Noddy mice. They lack inner-ear function, i.e., they are unable to hear and spin around while displaying head bobbing as they cannot distinguish up from down, left from right head movements. The cause of this behavior was unknown, until a group of geneticists lead by Kumar Alagramam identified a possible culprit: the protein protocadherin-15 of Noddy mice had one amino-acid changed (mutated) compared to the protein produced by their healthy relatives. Yet the puzzle remained: how a mutation that changed a single amino-acid (out of thousands) could cause such devastating effects? Our work demonstrated that this mutation prevented a handshake-like binding interaction between protocadherin-15 and cadherin-23 that is critical for inner-ear function and hair cell bundle integrity (see Figure adapted from Geng et al. 2013). The experiments and mouse model confirmed the relevance of the handshake interaction in vivo, and open the door to explore molecular therapies that could restore inner-ear function in Noddy mice and humans with similar mutations (see more in “Noddy, a mouse harboring a missense mutation in protocadherin-15, reveals the impact of disrupting a critical interaction site between tip-link cadherins in inner ear hair cells” The Journal of Neuroscience, 33:4395-4404, 2013).
We use our inner ear in everyday life, all the time. Not only to hear the world around us, but also to detect motion of our head, to maintain our gaze, and to discern up from down. Could you imagine going around the world without the senses of hearing and balance? That is exactly what happens to Noddy mice. They lack inner-ear function, i.e., they are unable to hear and spin around while displaying head bobbing as they cannot distinguish up from down, left from right head movements. The cause of this behavior was unknown, until a group of geneticists lead by Kumar Alagramam identified a possible culprit: the protein protocadherin-15 of Noddy mice had one amino-acid changed (mutated) compared to the protein produced by their healthy relatives. Yet the puzzle remained: how a mutation that changed a single amino-acid (out of thousands) could cause such devastating effects? Our work demonstrated that this mutation prevented a handshake-like binding interaction between protocadherin-15 and cadherin-23 that is critical for inner-ear function and hair cell bundle integrity (see Figure adapted from Geng et al. 2013). The experiments and mouse model confirmed the relevance of the handshake interaction in vivo, and open the door to explore molecular therapies that could restore inner-ear function in Noddy mice and humans with similar mutations (see more in “Noddy, a mouse harboring a missense mutation in protocadherin-15, reveals the impact of disrupting a critical interaction site between tip-link cadherins in inner ear hair cells” The Journal of Neuroscience, 33:4395-4404, 2013).
Cadherin tip-link bond in HD
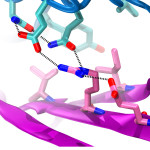 Hair cells of the inner ear are exquisitely sensitive and robust devices. Like microphones, they transform complex mechanical stimuli (sound and head movement) into an electrical signal that can be understood by our brain. At the core of this process, termed mechanotransduction, are hair-cell tip links, fine filaments made of protocadherin-15 and cadherin-23 that pull-open transduction channels. We have used X-ray crystallography, biochemical experiments, and molecular dynamics simulations to determine how these proteins interact with each other, to measure their strength and elasticity computationally, and to establish how deafness mutations would affect their biophysical properties. We obtained the first crystal structures of the interacting tips of both protocadherin-15 and cadherin-23. The complex forms a novel overlapping, antiparallel dimer in a handshake arrangement, different from that observed in interfaces of classical cadherins. Simulations indicated that cadherin repeats are stiff and the complex is stable in the presence of bound calcium. Simulations also provided “HD movies” of bond rupture and predicted the mechanical strength of the cadherin bond, which might be correlated with the threshold for noise-induced hearing loss. In addition, our data showed how deafness mutations would directly impair binding, would affect rigidity and decrease affinity for calcium, and would indirectly affect unfolding strength of tip-link EC repeats. Finally, this first set of structures of a heterophilic cadherin complex changes the classical view of cadherin interactions and opens the door to re-classify the whole cadherin family to search for other heterophilic complexes (see more in “Structure of a Force-Conveying Cadherin Bond Essential for Inner-Ear Mechanotransduction” Nature, 492:128-132, 2012 and “Structural Determinants of Cadherin-23 Function in Hearing and Deafness” Neuron, 66:85-100, 2010).
Hair cells of the inner ear are exquisitely sensitive and robust devices. Like microphones, they transform complex mechanical stimuli (sound and head movement) into an electrical signal that can be understood by our brain. At the core of this process, termed mechanotransduction, are hair-cell tip links, fine filaments made of protocadherin-15 and cadherin-23 that pull-open transduction channels. We have used X-ray crystallography, biochemical experiments, and molecular dynamics simulations to determine how these proteins interact with each other, to measure their strength and elasticity computationally, and to establish how deafness mutations would affect their biophysical properties. We obtained the first crystal structures of the interacting tips of both protocadherin-15 and cadherin-23. The complex forms a novel overlapping, antiparallel dimer in a handshake arrangement, different from that observed in interfaces of classical cadherins. Simulations indicated that cadherin repeats are stiff and the complex is stable in the presence of bound calcium. Simulations also provided “HD movies” of bond rupture and predicted the mechanical strength of the cadherin bond, which might be correlated with the threshold for noise-induced hearing loss. In addition, our data showed how deafness mutations would directly impair binding, would affect rigidity and decrease affinity for calcium, and would indirectly affect unfolding strength of tip-link EC repeats. Finally, this first set of structures of a heterophilic cadherin complex changes the classical view of cadherin interactions and opens the door to re-classify the whole cadherin family to search for other heterophilic complexes (see more in “Structure of a Force-Conveying Cadherin Bond Essential for Inner-Ear Mechanotransduction” Nature, 492:128-132, 2012 and “Structural Determinants of Cadherin-23 Function in Hearing and Deafness” Neuron, 66:85-100, 2010).
Simulations of membrane voltage with applied electric fields: Patch clamping in silico
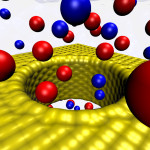 The electric potential difference across biological membranes plays a central role in varied and essential biological processes such as ATP production and the transmission of nerve impulses. Accurately accounting for this membrane potential is a critical aspect that requires consideration in the case of realistic simulations of biological membrane systems. A widely used (and sometimes misused) treatment of the membrane potential in molecular dynamics consists of applying an external uniform electric field E perpendicular to the membrane. The field acts on all charged particles throughout the simulated system, and the resulting applied membrane potential V is equal to the applied electric field times the length of the periodic cell in the direction perpendicular to the membrane. A series of test simulations based on simple membrane-slab models were carried out to clarify the consequences of the applied field. These illustrative tests demonstrated that the constant-field method is a simple and valid approach for accounting for the membrane potential and perform “patch clamp experiments” in silico (text adapted from “Constant Electric Field Simulations of the Membrane Potential Illustrated with Simple Systems” BBA – Biomembranes, 1818:294-302, 2012).
The electric potential difference across biological membranes plays a central role in varied and essential biological processes such as ATP production and the transmission of nerve impulses. Accurately accounting for this membrane potential is a critical aspect that requires consideration in the case of realistic simulations of biological membrane systems. A widely used (and sometimes misused) treatment of the membrane potential in molecular dynamics consists of applying an external uniform electric field E perpendicular to the membrane. The field acts on all charged particles throughout the simulated system, and the resulting applied membrane potential V is equal to the applied electric field times the length of the periodic cell in the direction perpendicular to the membrane. A series of test simulations based on simple membrane-slab models were carried out to clarify the consequences of the applied field. These illustrative tests demonstrated that the constant-field method is a simple and valid approach for accounting for the membrane potential and perform “patch clamp experiments” in silico (text adapted from “Constant Electric Field Simulations of the Membrane Potential Illustrated with Simple Systems” BBA – Biomembranes, 1818:294-302, 2012).
Mechanosensitive channels – MscS
 Mechanosensitive channels are membrane proteins that open and close in response to mechanical forces, thus generating an ionic current that eventually becomes an electrical signal or that simply helps in the regulation of cell volume. In bacteria, a protein called MscS prevents cell burst upon osmotic shocks by controlling passage of water and ions in response to membrane tension. Mechanical channel gating and transport of ions through this protein were studied by combining different experimental and computational methodologies: patch-clamp experiments and electron paramagnetic resonance (EPR) measurements (Perozo’s Lab), all-atom molecular dynamics simulations (Schulten’s Lab), a coarse-grained model (Ravaioli’s Lab), and restrained molecular dynamics (Roux’s Lab). The experiments revealed that MscS can be found in inactive, closed, or open conformations, while the MscS simulations correctly predicted that the first X-ray crystal structure of MscS is not that of a completely open, non-selective channel. Finally, restrained molecular dynamics incorporating data from EPR experiments provided a model for the closed and open conformations of MscS in its native environment. These studies have helped to consolidate a model for MscS gating involving tilting of helices and pore enlargement induced by membrane tension, and to suggest a general mechanism for lipid-mediated channel gating. Importantly, the simulations were essential to establish the conduction state of the first MscS structure and trigger the search for wider, truly open states (text adapted from http://www.ks.uiuc.edu/Research/MscSchannel/).
Mechanosensitive channels are membrane proteins that open and close in response to mechanical forces, thus generating an ionic current that eventually becomes an electrical signal or that simply helps in the regulation of cell volume. In bacteria, a protein called MscS prevents cell burst upon osmotic shocks by controlling passage of water and ions in response to membrane tension. Mechanical channel gating and transport of ions through this protein were studied by combining different experimental and computational methodologies: patch-clamp experiments and electron paramagnetic resonance (EPR) measurements (Perozo’s Lab), all-atom molecular dynamics simulations (Schulten’s Lab), a coarse-grained model (Ravaioli’s Lab), and restrained molecular dynamics (Roux’s Lab). The experiments revealed that MscS can be found in inactive, closed, or open conformations, while the MscS simulations correctly predicted that the first X-ray crystal structure of MscS is not that of a completely open, non-selective channel. Finally, restrained molecular dynamics incorporating data from EPR experiments provided a model for the closed and open conformations of MscS in its native environment. These studies have helped to consolidate a model for MscS gating involving tilting of helices and pore enlargement induced by membrane tension, and to suggest a general mechanism for lipid-mediated channel gating. Importantly, the simulations were essential to establish the conduction state of the first MscS structure and trigger the search for wider, truly open states (text adapted from http://www.ks.uiuc.edu/Research/MscSchannel/).
Elasticity of repeat proteins – Ankyrin
 The process of transforming a mechanical stimulus into an electrical or biochemical signal is ubiquitous in living organisms. This transformation is called “mechanotransduction” and is accomplished by a series of amazingly minute devices that often connect a soft spring to an ion channel. Mechanotransduction ion channel candidates are likely to contain subunits of the TRP channel family, like TRPN1 (NOMPC), which has an extended N-termini with 29 ankyrin repeats. Molecular dynamics simulations showed that these ankyrin repeats could act as a soft gating spring essential for channel activation. The mechanical response of ankyrin proteins was characterized at low-force by an elastic behavior reflecting changes of the overall shape (tertiary structure elasticity, TSE). Simulations also showed that at high-force their mechanical response was characterized by elasticity stemming from sequential unraveling of secondary structure elements (secondary structure elasticity, SSE). Both tertiary and secondary structure elasticity of ankyrin repeats, predicted first through simulations, have been confirmed by independent AFM experiments. Our work on ankyrin repeats pioneered the investigation of the elasticity of repeat proteins (now extended to other types of repeat proteins); it provided a framework to interpret and guide AFM experiments; and it introduced the concept of tertiary structure elasticity. However, the role played by ankyrin elasticity in vivo remains untested (text adapted from http://www.ks.uiuc.edu/Research/hearing/).
The process of transforming a mechanical stimulus into an electrical or biochemical signal is ubiquitous in living organisms. This transformation is called “mechanotransduction” and is accomplished by a series of amazingly minute devices that often connect a soft spring to an ion channel. Mechanotransduction ion channel candidates are likely to contain subunits of the TRP channel family, like TRPN1 (NOMPC), which has an extended N-termini with 29 ankyrin repeats. Molecular dynamics simulations showed that these ankyrin repeats could act as a soft gating spring essential for channel activation. The mechanical response of ankyrin proteins was characterized at low-force by an elastic behavior reflecting changes of the overall shape (tertiary structure elasticity, TSE). Simulations also showed that at high-force their mechanical response was characterized by elasticity stemming from sequential unraveling of secondary structure elements (secondary structure elasticity, SSE). Both tertiary and secondary structure elasticity of ankyrin repeats, predicted first through simulations, have been confirmed by independent AFM experiments. Our work on ankyrin repeats pioneered the investigation of the elasticity of repeat proteins (now extended to other types of repeat proteins); it provided a framework to interpret and guide AFM experiments; and it introduced the concept of tertiary structure elasticity. However, the role played by ankyrin elasticity in vivo remains untested (text adapted from http://www.ks.uiuc.edu/Research/hearing/).
Elasticity and dynamics of cadherins
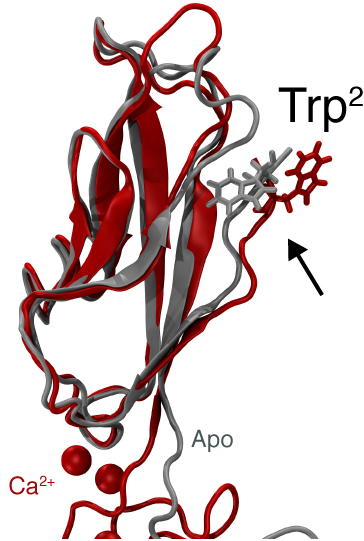 Adhesion between human cells organizes our body into its organs and parts. The adhesion comes about through an intricate system of molecules that perform their task in a highly selective manner such that the body’s different types of cells will find the right cells and stick to them. This selectivity leads to tissue differentiation and the organization of organs as complicated as the brain. Cadherin proteins form a particular family of such adhesion molecules. Interestingly, they glue cells together only in the presence of calcium. Large-scale all-atom molecular dynamics simulations revealed that in the absence of calcium cadherin behaves as a loose and weak rope. In the presence of calcium, however, cadherin molecules turn into stiff hooks that link cells together. The simulations also revealed how calcium ions protect cadherins from unfolding when large mechanical forces are applied, and how they regulate the availability of key tryptophan residues involved in bond formation. These findings have been confirmed by independent computational studies and experiments (text adapted from http://www.ks.uiuc.edu/Research/cadherin/).
Adhesion between human cells organizes our body into its organs and parts. The adhesion comes about through an intricate system of molecules that perform their task in a highly selective manner such that the body’s different types of cells will find the right cells and stick to them. This selectivity leads to tissue differentiation and the organization of organs as complicated as the brain. Cadherin proteins form a particular family of such adhesion molecules. Interestingly, they glue cells together only in the presence of calcium. Large-scale all-atom molecular dynamics simulations revealed that in the absence of calcium cadherin behaves as a loose and weak rope. In the presence of calcium, however, cadherin molecules turn into stiff hooks that link cells together. The simulations also revealed how calcium ions protect cadherins from unfolding when large mechanical forces are applied, and how they regulate the availability of key tryptophan residues involved in bond formation. These findings have been confirmed by independent computational studies and experiments (text adapted from http://www.ks.uiuc.edu/Research/cadherin/).
Elasticity and dynamics of fibrinogen
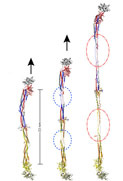 Blood clots are a necessary response to prevent bleeding due to mechanical injury. These clots must be both elastic enough to resist the sheering forces of blood flow but also stiff enough to not rupture before the underlying tissue has healed. A combination of experiments (Lim’s lab) and simulations (Schulten’s lab) demonstrated the presence of a force plateau when stretching fibrinogen, and related the plateaus to an ordered, stepwise unfolding of the protein’s coiled-coil domains. A triphasic response to stretching was seen in single molecules as well as in polymerized fibrin monomers. This response should play a significant role in the mechanical response of fibrin to blood’s shear forces, critical to its biological function in maintaining the integrity of blood clots (text adapted from http://www.ks.uiuc.edu/Research/fibrinogen/).
Blood clots are a necessary response to prevent bleeding due to mechanical injury. These clots must be both elastic enough to resist the sheering forces of blood flow but also stiff enough to not rupture before the underlying tissue has healed. A combination of experiments (Lim’s lab) and simulations (Schulten’s lab) demonstrated the presence of a force plateau when stretching fibrinogen, and related the plateaus to an ordered, stepwise unfolding of the protein’s coiled-coil domains. A triphasic response to stretching was seen in single molecules as well as in polymerized fibrin monomers. This response should play a significant role in the mechanical response of fibrin to blood’s shear forces, critical to its biological function in maintaining the integrity of blood clots (text adapted from http://www.ks.uiuc.edu/Research/fibrinogen/).
Molecular dynamics simulations of melting in infinite crystalline solids
 Melting of an infinite solid in two dimensions has been described as a process driven by a proliferation of thermally excited dislocation pairs in the Kosterlitz-Thouless-Halperin-Nelson-Young (KTHNY) theory. The theory, formulated at vanishing external pressure, predicts the existence of a new, “hexatic,” intermediate thermodynamic phase. Melting is predicted to start with a second-order phase transition from the crystalline to the hexatic phase, at which point there is a universal jump of a normalized elastic constant from a finite value to 0. This is followed by a second transition from the hexatic to the liquid phase at a higher temperature. Working with Daniel Asenjo, Simon Poblete, Rodrigo Soto and Fernando Lund, we used molecular dynamics simulations to study the melting transition in two dimensions. We found a single-step solid-to-liquid transition when a vanishing external pressure was applied to the system, in contrast to a multistep transition with an hexatic phase observed at high pressure. However, within a narrow temperature interval defining the solid-to-liquid transition at zero external pressure, the monitored relaxation times, elastic constants, and evolution of the number of dislocations were all consistent with the KTHNY theory. We suggest that, because of the necessary interplay between many length scales, a stable hexatic phase will be unambiguously observed only in systems with at least ∼10^6 particles at zero external pressure (text adapted from “Characterization of the melting transition in two dimensions at vanishing external pressure using molecular dynamics simulations” Physical Review B, 83:174110, 2011).
Melting of an infinite solid in two dimensions has been described as a process driven by a proliferation of thermally excited dislocation pairs in the Kosterlitz-Thouless-Halperin-Nelson-Young (KTHNY) theory. The theory, formulated at vanishing external pressure, predicts the existence of a new, “hexatic,” intermediate thermodynamic phase. Melting is predicted to start with a second-order phase transition from the crystalline to the hexatic phase, at which point there is a universal jump of a normalized elastic constant from a finite value to 0. This is followed by a second transition from the hexatic to the liquid phase at a higher temperature. Working with Daniel Asenjo, Simon Poblete, Rodrigo Soto and Fernando Lund, we used molecular dynamics simulations to study the melting transition in two dimensions. We found a single-step solid-to-liquid transition when a vanishing external pressure was applied to the system, in contrast to a multistep transition with an hexatic phase observed at high pressure. However, within a narrow temperature interval defining the solid-to-liquid transition at zero external pressure, the monitored relaxation times, elastic constants, and evolution of the number of dislocations were all consistent with the KTHNY theory. We suggest that, because of the necessary interplay between many length scales, a stable hexatic phase will be unambiguously observed only in systems with at least ∼10^6 particles at zero external pressure (text adapted from “Characterization of the melting transition in two dimensions at vanishing external pressure using molecular dynamics simulations” Physical Review B, 83:174110, 2011).
Role of photoevaporation in the dispersal of viscous circumstellar disks
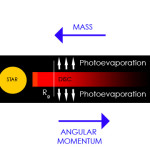 The processes responsible for dispersing the disks around young stars are poorly understood. This issue is important inasmuch as it impacts on the time available for planet formation in circumstellar disks. Working with A. Gendrine under the supervision of Dr. C. J. Clarke, we explored ultraviolet switch models for the dispersal of circumstellar disks in T Tauri stars that involve both photoevaporation by the central star and viscous evolution. We showed that in combination these processes generate the observed ‘two-time-scale’ behavior for the dispersal of such disks, whereby the disk is rapidly dispersed at the end of its life on a time-scale that is a small fraction of the disk lifetime (text adapted from “The dispersal of circumstellar discs: the role of the ultraviolet switch” Monthly Notices of the Royal Astronomical Society, 328:485-491, 2001).
The processes responsible for dispersing the disks around young stars are poorly understood. This issue is important inasmuch as it impacts on the time available for planet formation in circumstellar disks. Working with A. Gendrine under the supervision of Dr. C. J. Clarke, we explored ultraviolet switch models for the dispersal of circumstellar disks in T Tauri stars that involve both photoevaporation by the central star and viscous evolution. We showed that in combination these processes generate the observed ‘two-time-scale’ behavior for the dispersal of such disks, whereby the disk is rapidly dispersed at the end of its life on a time-scale that is a small fraction of the disk lifetime (text adapted from “The dispersal of circumstellar discs: the role of the ultraviolet switch” Monthly Notices of the Royal Astronomical Society, 328:485-491, 2001).