Our Projects
Actin: its friends and foes
Actin is one of the most abundant and functionally versatile proteins on the planet. As a track for myosin-based motility, actin governs muscle contraction, organelle transport, and closure of the contractile ring. Actin directional polymerization serves motor purposes and thus drives exo- and endocytosis, cell division, migration, invasion (e.g., by immune and cancer cells), and other cellular processes. Currently, our interests in this area are focused on the directions outlined below.
Actin is involved in the pathogenesis of infectious diseases as an element of the innate immune system, but also as a target that can be hijacked by many bacterial and viral toxins. We are interested in deciphering the role of actin in both phenomena.
Plastin isoforms (Friends)
- All three plastin/fimbrin isoforms bundle actn filaments in a Ca(2+)-dependent manner. Mutations in T-plastin result in congenital osteoporosis and diaphragmatic hernia. Mutations in I-plastin lead to hearing loss. Expression of L-plastin is limited to immune cells in health, but is frequently elevated in tumors and associated with their increased metastatic activity. We compare properties of all plastin isoforms to undestand their normal functions and role in various diseases.
ChatGPT’s poem about Plastins:
Plastin, a protein so fine, Allosterically regulated, with great design. Its activity, controlled by change, A master of function, with a range.
It bends and twists, with ease, In response to signals, with great tease. Its shape, a key to its control, Allowing it to fulfill its role.
Plastin, a scaffold so strong, Supporting cells, all day long. It holds the cytoskeleton in place, Withstanding the forces of the race.
But its versatility, what sets it apart, Its ability to respond, with a smart start. A protein of many talents, it’s clear, Allosterically regulated, with great cheer.
So let us not forget this gem, Plastin, a protein, with a rich stem. For it plays a vital role, in the cell, Supporting life, with its allosteric swell.
Actin-specific toxins from Vibrio and Salmonella spp. (Foes)
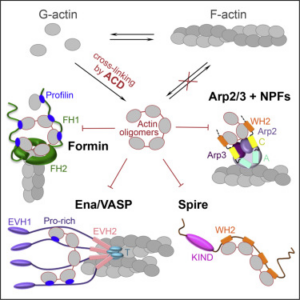
ACD covalently cross-links actin subunits into polymerization-defective oligomers and thus was considered to be a G-actin sequestering toxin. Instead, we found that the ACD-produced actin oligomers that bind with high affinity and potently block the activity of numerous actin organizers that contain several G-actin binding sites. These proteins include formin, Ena/VASP, NPF, Spire, Cobl, and other protein families. In this host-pathogen battle, ACD acts as a smart spy, whic instead of killing all the solders (actin monomers) converts some of them into traitors (actin oligomers), who effectively neutralize the officers (actin organizing proteins), bringing disorder and chaos into the host’s army.
Such an effective strategy allows achieve maximal goals by minimal means. Disorganization of the cytoskeleton neutralizes the motility and phagocytosis in immune cells and disrupts the integrity of epithelial barriers.
 VopF/VopL toxins of Vibrio cholerae/Vibrio parahaemolyticus: actors of “impossible” (Foes).
VopF/VopL toxins of Vibrio cholerae/Vibrio parahaemolyticus: actors of “impossible” (Foes).
A key property of actin filaments is their polarity. In the cell, actin elongation is encouraged at the barbed (plus) ends, but blocked at the pointed (minus) ends. This separation is achieved by propertied of the filament and coordinated action of actin binding proteins (ABPs). We discovered that VopF/VopL toxins move inside the cell by promoting processive polymerization at the minus ends of the filaments – process that thought to be impossible. The detailed mechanism of this one-of-a-kind process is currently under investigation.
Selective targeting and elimination of cancer cells with bacterial toxins
- Several bacterial toxins have attracted significant interest as potent antitumor agents due to their selectivity, ability to penetrate the cell membrane, high resistance against host defense systems, and demonstrated capacity to kill cells by mechanisms independent of the drug-resistant phenotypes of most tumors. Yet, the specificity of the currently recognized toxins towards tumor cells is limited due to a broad cross-specificity of the toxin receptors. We propose to deliver split variants of potent bacterial toxins via two different pathways uniquely represented only on the surface of cancer cells. The idea is that the fully functional toxin will be assembled only in the cytoplasm of a doubly targeted cancer cell.
Selective inactivation of bacterial toxins by human defensins
- Defensins are a family of short cationic immune peptides with a broad repertoire of anti-microbial activities. Defensins disorganize bacterial cell membranes and inactivate protein bacterial toxins while showing little effect on host’s proteins. Yet, the amazing selectivity of defensins against various unrelated toxins is not well understood, nor has a coherent unifying hypothesis been proposed to explain this selectivity. We have developed a keen interest in deciphering the mechanisms of amazingly selective inact
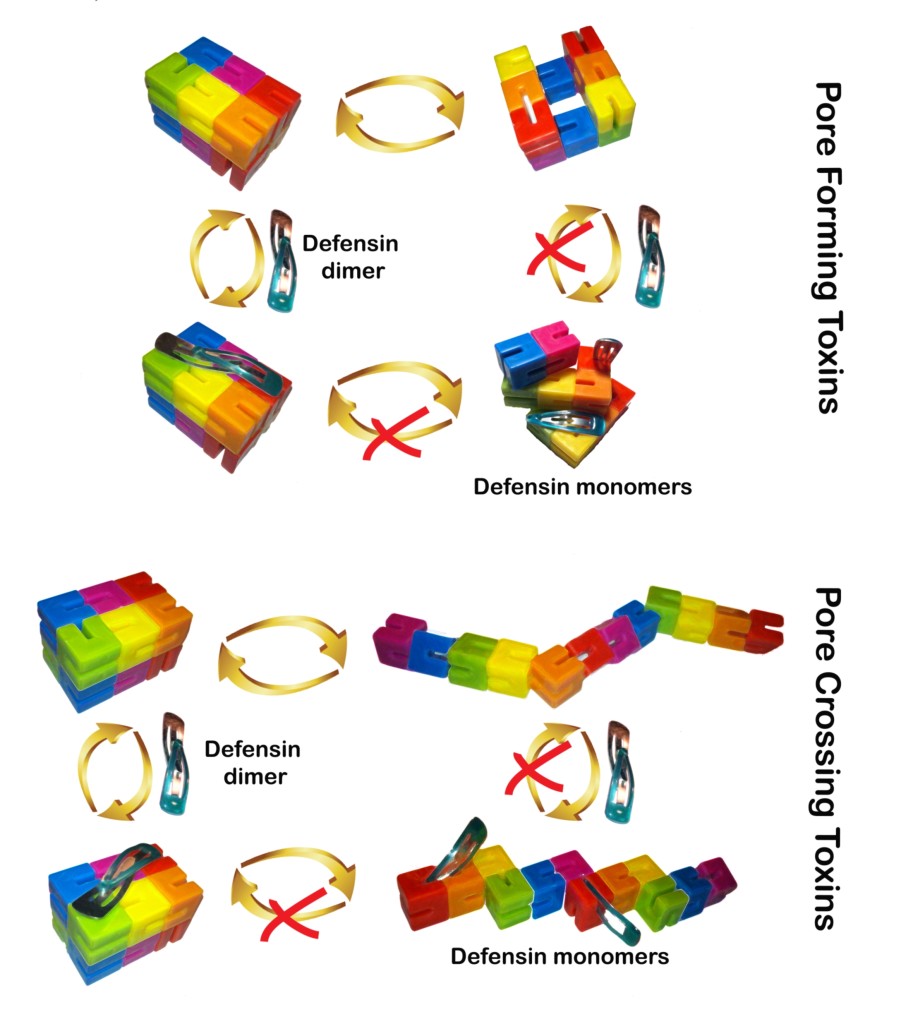 ivation of bacterial toxins by human defensins. We propose that many bacterial toxins share an elusive common property that can be efficiently exploited by human defensins and we are currently in the process of identification of these elusive properties. The data acquired in the course of the project should lead to the development of rationally designed anti-toxin therapeutic agents.
ivation of bacterial toxins by human defensins. We propose that many bacterial toxins share an elusive common property that can be efficiently exploited by human defensins and we are currently in the process of identification of these elusive properties. The data acquired in the course of the project should lead to the development of rationally designed anti-toxin therapeutic agents.
To accomplish our goals we employ highly interdisciplinary combination of biochemical, biophysical, and cell biology approaches, including but not limited to methods of fluorescence spectroscopy, circular dichroism, calorimetry, mass spectrometry, electron microscopy, cell imaging, fluorescent microscopy, and others. In addition, we collaborate with several groups for X-ray crystallography, NMR, and computational biology experimentsthe