Modern chemistry enables us to create any molecule that we might imagine to be of benefit to our society. This allows us to accomplish such feats as converting: fossil fuels into cancer therapies, corn stalks into biofuels, or sugar cane into tracers for mapping the human brain. Nonetheless, while we can theoretically synthesize nearly any complex architecture, it is often prohibitively challenging to do so. Obstacles range from the limited variety and availability of petroleum-derived precursors to the inefficiency and non-scalability of multi-step synthetic sequences.
The Nagib Laboratory seeks to bridge the gap between what is possible and practical in the realm of organic synthesis. Our goal is to expand the synthetic toolbox by designing fundamentally new activation strategies and catalysts. We aim to harness the untapped reactivity of cheap and abundant chemical feedstocks as well as enable the late-stage functionalization of complex natural products. We are currently inventing multi-faceted approaches for selective C-H and C-O activation, using combinations of radical (1e-) and closed shell (2e-) processes. By emphasizing the design of novel, dual-catalytic strategies (organometallic, organocatalytic, redox-neutral, etc.) and a careful elucidation of their unique mechanisms, we are developing useful methodologies to enable non-classical synthetic disconnections. Our chemistry has important applications in various interdisciplinary arenas, including the streamlined synthesis of improved medicines, materials, and biofuels.
Harnessing Radicals
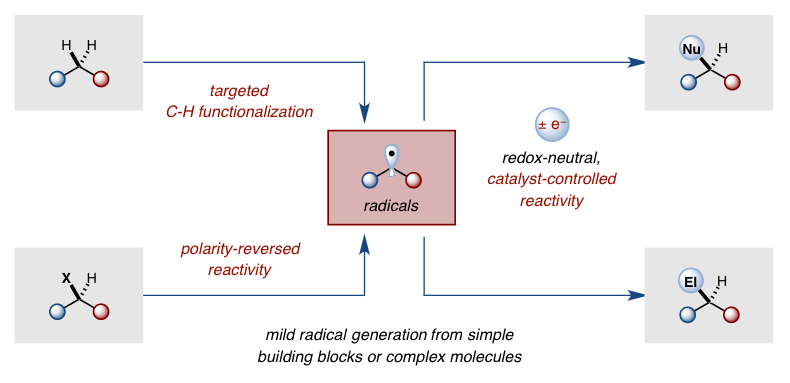
The frequent need for harsh chemical or photolytic conditions to generate radicals has created the misconception that radical chemistry is incompatible with complex organic synthesis. Our lab is developing new techniques for mild generation of open shell intermediates in order to demonstrate that radical reactivity can be harnessed as an important tool to enable useful synthetic disconnections that are either complementary (umpolung) with, or difficult to achieve (C-H activation) by, two-electron processes.
C-H Activation
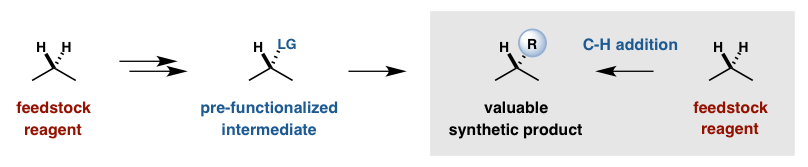
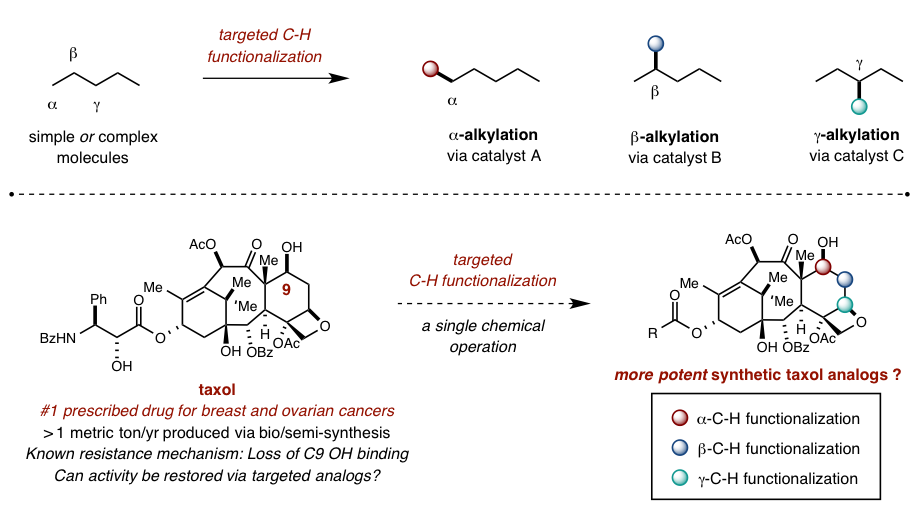
Recent innovations in organometallic and radical-based C-H activation strategies have demonstrated that this most ubiquitous (non)functional group can be exploited to introduce synthetic complexity to simple molecules in a more direct and efficient manner. Our lab is building on the rare and exciting examples of selective C-H functionalization by developing a toolbox of catalysts that can introduce new functional groups at any desired position of a simple or complex molecule. By replacing substrate-based selectivity with catalyst-directed programming, classic retrosynthetic analysis can be complemented by at-will functionalization of late-stage synthetic intermediates as well as biologically-derived natural products.
C-O Activation
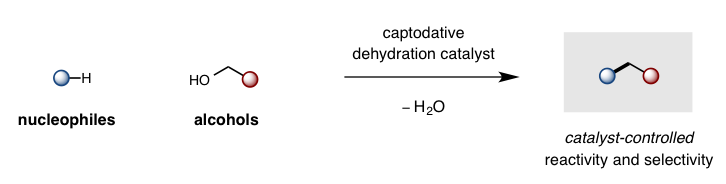
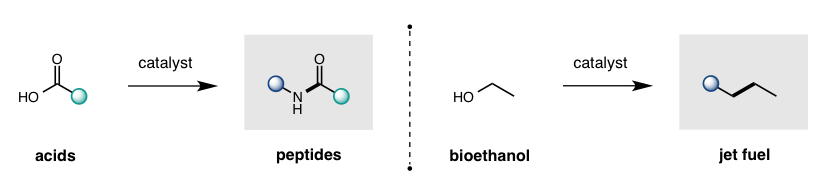
While cleavage of a C-O bond is one of the most common retrosynthetic disconnections in organic synthesis (e.g. Mitsunobu displacement of alcohols, peptide coupling of carboxylic acids), there is a significant dearth in catalytic strategies to displace an -OH group – forcing chemists to rely on wasteful, stoichiometric activation before desired C-O displacement. Our lab is designing captodative catalysts (with synergistic push-pull electronics) to enable the thermodynamically favorable displacement of H2O as a sole, environmentally-friendly byproduct. This approach enables catalytic access to some of the most frequently employed and valuable transformations in organic synthesis.
The Nagib group is always looking for enthusiastic and motivated graduate and undergraduate students interested in developing new areas of catalysis.
