Artificial Metalloenzymes
Artificial Metalloenzymes for Small Molecule Activation Reactions
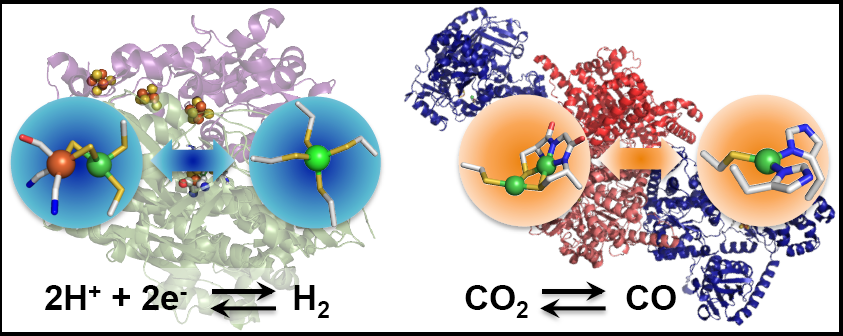
Nickel-containing metalloenzymes are highly relevant in the context of energy conversion reactions and environmental issues, as they play important roles in regulating global gas cycles. For example, the [NiFe] hydrogenase (H2ase) carries out both hydrogen evolution and oxidation with high rates and thermodynamic efficiencies. Carbon monoxide dehydrogenase (CODH) reversibly interconverts carbon dioxide and carbon monoxide, and acetyl coenzyme-A synthase (ACS) is responsible for carbon-carbon bond forming reactions required for synthesis of acetyl CoA, a key metabolite for anaerobic bacteria in the Wood-Ljungdahl pathway. Thus, systems such as these can offer solutions to present-day energy challenges and climate issues. However, complex enzymes such as H2ase and ACS lack the stability to be extensively studied at the molecular level or implemented on a large scale into devices, limiting their utility.
We are working to overcome the limitations of fragile enzyme systems using small metalloproteins as platforms for redesign. By harnessing the advantages of stable metal-binding centers and well-defined secondary sphere interactions, we are developing robust protein-based models of highly active enzymes such as H2ase and the tightly coupled CODH/ACS complex. Recent work in our lab has demonstrated high levels of hydrogenase-like activity in nickel-substituted rubredoxin (NiRd) via solution-phase and electrocatalytic assays. We have also developed the first protein-based model of the proximal nickel center in ACS using nickel-substituted azurin. A suite of biophysical techniques, including vibrational, electronic, and magnetic spectroscopies along with electrochemistry are employed to probe the structures, activities, and catalytic mechanisms of these engineered metalloenzymes.
A valuable aspect of “bioinorganic chemistry” involves the development of molecular mimics of large enzyme complexes. In addition to studies on protein-based models, we are using spectroscopic and theoretical methods to characterize synthetic models of metalloenzymes such as hydrogenase. This research will provide important metrics for comparison between synthetic and natural enzyme systems. Taken together, these projects will provide insight into the challenging catalytic reactions carried out both by the engineered and native systems and facilitate design of highly active artificial metalloenzymes.
Heterobimetallic Oxidases
Metal Binding and Cofactor Assembly in Heterobimetallic Mn/Fe Oxidases
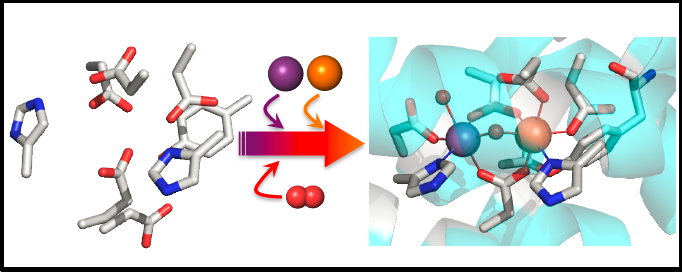
Across the biological (and even abiological) world, multimetallic active sites carry out key biochemical reactions, from metal homeostasis and gas regulation to metabolism and cellular signaling. Examples of these sites can be found in proteins ranging from nitrogenase to cytochrome c oxidase, highlighting their diversity. Heterobimetallic cofactors represent an important subset of these active sites, combining the properties of two different metals to access new chemistry. Recently, a novel heterobimetallic protein was identified in several pathogens and extremophiles, including Mycobacterium tuberculosis. This protein spontaneously incorporates a Mn/Fe cofactor under aerobic conditions and therefore has been designated “R2lox”, or “R2-like ligand-binding oxidase,” in reference to the class of ribonucleotide reductase R2 subunits that have been found to require Mn/Fe cofactors for activity.
Due to their intrinsic metal specificity, the R2lox proteins offer a unique opportunity to study the thermodynamics and kinetics of metal binding and cofactor assembly. Despite identical primary coordination spheres, manganese is selectively taken up into one site, and iron into the other, when both metals are present. This observation runs counter to the well-known Irving-Williams series for divalent metals, which states that Mn(II) complexes are more labile, and therefore less stable, than Fe(II) complexes. Two additional observations have garnered further interest in the R2lox proteins: i) A long-chain fatty acid has been isolated within the protein, suggesting a role for R2lox in lipid metabolism; and ii) upon metal binding and oxygen activation, a covalent tyrosine-valine crosslink within the protein interior is formed. This represents the first example of two-electron redox chemistry performed by a Mn/Fe cofactor and extends potential utility of these systems towards oxidase and oxygenase activity.
We are interested in probing the structure, kinetics, and mechanism of assembly of the heterobimetallic Mn/Fe R2lox proteins. To do this, several biophysical techniques are employed, including steady state and time-resolved resonance Raman and optical spectroscopies, in conjunction with biochemical approaches. By studying this system, we hope to gain important insight into the factors governing substrate binding within metalloproteins and the reactivity of Mn/Fe cofactors as well as explore potential opportunities for manipulating these elements. Additionally, understanding the role of R2lox in the pathogenicity of organisms such as M. tuberculosis can provide novel avenues for development of selective therapeutics targeting this class of enzyme.
CGRRS
Computationally-Guided Resonance Raman Spectroscopy (CGRRS)
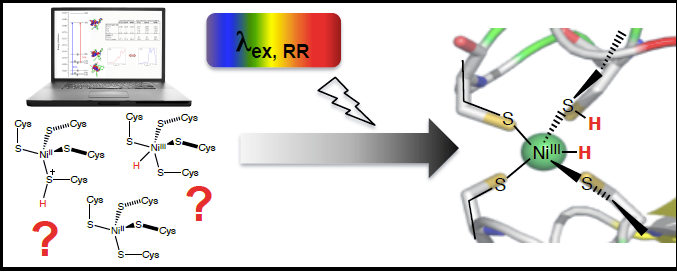
The general paradigm that structure is correlated to function and mechanism is widely accepted across scientific disciplines. However, in complex enzymatic systems, such as those involved in small molecule activation and energy conversion reactions, the requisite presence of multiple cofactors often obscures conventional spectroscopic signatures. Spectral congestion can be avoided by using selective experimental techniques, such as resonance Raman (RR) spectroscopy, provided it is possible to identify the appropriate conditions under which selectivity can be achieved. Towards this end, we are applying quantum chemical calculations to inform experimental design for application of site-specific RR spectroscopy, a technique we have called “Computationally-guided resonance Raman spectroscopy (CGRRS)”. These predictions are evaluated experimentally using a custom-built, highly tunable RR system capable of accessing excitation wavelengths throughout the UV, visible, and near-IR wavelengths.
We are using CGRRS to investigate a variety of biologically relevant proteins and their variants. Spectroelectrochemical studies on model compounds complement the enzymatic studies. By correlating the predicted RR spectra of proposed intermediates to experimental results, CGRRS enables structural determination of critical intermediates. This approach can provide mechanistic information on a number of enzymatic reactions, including proton reduction, CO2 reduction, nitrogen fixation, water oxidation, and oxygen reduction.