Overview
Our research spans from the mechanistic study of biological phenomena, development of novel methodologies, to applications of the mechanistic understanding and methodologies to discover novel therapeutic agents and chemical probes. It is estimated that ~80% of all disease-relevant human proteins are undruggable by current drug modalities, which include small molecules (molecular weight <500) and biologics (molecular weight >5000). Prominent examples of undruggable proteins are those involved in intracellular protein-protein interactions (PPIs) and the defective/missing proteins caused by genetic mutations. The ultimate goal of our research is to find a general strategy for drugging these challenging targets. The following projects are under current investigation in our group.
-
How Do Biomolecules Cross the Cell Membrane? Most biomolecules (e.g., peptides, proteins, and nucleic acids) cannot travel across the cell membrane. Consequently, the cell membrane poses one of the biggest challenges in drug discovery. Nevertheless, viruses and an increasing number of folded proteins (e.g., bacterial toxins and certain eukaryotic proteins) are known to cross the cell membrane in both directions, but their mechanisms of action have been a longstanding mystery. We recently discovered a novel membrane translocation mechanism, vesicle budding and collapse (VBC), which drives the cellular entry of cell-penetrating peptides. We are currently investigating the potential involvement of VBC during the cellular entry of bacterial toxins and nonenveloped viruses as well as the unconventional secretion of folded proteins by eukaryotes. We are also leveraging our mechanistic understanding to design additional cell-penetrating peptides/proteins as more efficient drug delivery vehicles. Read More…
-
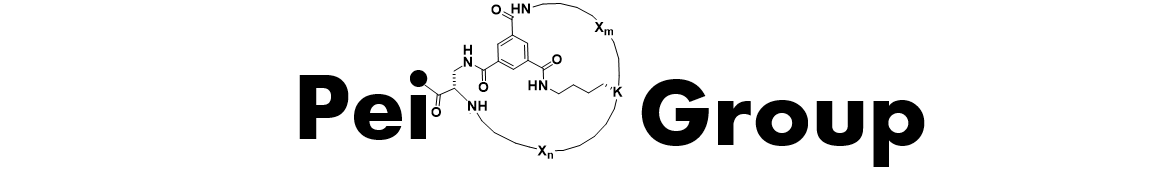
Macrocyclic Peptides as Protein-Protein Interaction Inhibitors. PPIs represent an exciting but also challenging class of drug targets, because they usually have large, flat binding sites, to which small molecules do not bind with high affinity or specificity. We and others have demonstrated that macrocyclic peptides in the molecular weight range of 500-2000 serve as effective PPI inhibitors. We also developed a powerful method to chemically synthesize and rapidly screen large libraries of macrocyclic peptides (up to 30 million different compounds) against essentially any protein of interest. We are currently applying this technology and medicinal chemistry strategies to discover macrocyclic peptide inhibitors against PPIs that cause human diseases (e.g., cancer, inflammation, and autoimmunity). Read More…
-
Development of Intracellular Biologics and Chemical Probes. Biologic drugs (e.g., monoclonal antibodies) have transformed the drug industry over the last few decades but are limited to extracellular targets. We are now integrating the drug discovery and delivery technologies described above to develop intracellular biologics as next-generation therapeutics. Our first approach involves the conjugation of cyclic cell-penetrating peptides to peptides, proteins, and nucleic acids to render the latter cell permeable and biologically active. In our second approach, cell-penetrating and target-binding peptide moieties are integrated into the same molecular structures (or hybrids) that are capable of efficiently entering the cell and engaging PPI targets. Finally, we have engineered a family of membrane translocation protein domains (MTDs) and are using them to deliver potentially any protein into mammalian and plant cells. Read More…
Major Techniques in Research:
-
Solid-phase peptide synthesis
-
Solution-phase synthesis of library building blocks and other small molecules.
-
High-throughput library screening and peptide sequencing by mass spectrometry.
-
Molecular cloning, expression, and purification of proteins.
-
Biophysical characterization of proteins (e.g., fluorescence polarization and surface plasmon resonance).
-
Enzyme kinetics and inhibition.
-
Mammalian tissue culture, transfection, co-immunoprecipitation, and western blotting.
-
Confocal microscopy and flow cytometry.
Current Sources of Research Funding: