Classical structural biology techniques such as X-ray crystallography and nuclear magnetic resonance provide great atomic details of the protein structure but they suffer from low throughput and challenge posed by the complexity and dynamics of proteins. Extending the application of mass spectrometry to generate information about protein quaternary structure and protein-protein interactions is beneficial because of its high throughput, low sample consumption, and capability of analyzing mixtures.
The ultimate goal of this research is to develop a better mass spectrometric tool to study protein quaternary structure in an efficient and effective manner. The commonly used neutral gas collision method, is usually believed to cause subunit unfolding during activation.The undesired structural change during activation makes it difficult to relate the structural information of the products to that of the precursor. In contrast, surface induced dissociation (SID) of protein complexes, originally developed in our laboratory, has shown promising results of generating subcomplex products which are representative of the native subunit arrangement.
Read more about SID of protein complexes and why it is different from CID
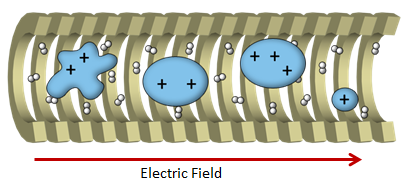
Ion mobility (IM) is gas-separation technique, where ions are driven by an electric field while traveling in bath gas. Larger ions interacts with bath gas more frequently; Low charged ions experience less force from the electric field. Thus ions are separated in drift time based on their size and charge. IM is able to distinguish different conformations of proteins, some of which could be transient and difficult to be studied with classical methods. Incorporation of IM gives the opportunity to separate different conformers of a protein complex, which could be relevant to their physiological functions, for subsequent structural elucidation by SID.
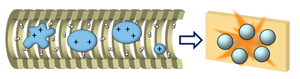
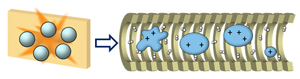
IM measurements of SID fragments allow us to study their conformation in order to improve our knowledge of the SID mechanism. This will further help us understand dissociation behavior of protein complexes so that we will be able to better utilize the data from gas-phase experiments to elucidate native structures in solution.
Related Publications
Zhou, M.; Huang, C.; Wysocki, V. H., Surface-Induced Dissociation of Ion Mobility-Separated Noncovalent Complexes in a Quadrupole/Time-of-Flight Mass Spectrometer. Anal. Chem. (Washington, DC, U. S.) 2012, 84 (14), 6016-6023.
Zhou, M.; Dagan, S.; Wysocki, V. H., Protein subunits released by surface collisions of noncovalent complexes: nativelike compact structures revealed by ion mobility mass spectrometry. Angew Chem Int Ed 2012, 51 (18), 4336-9.
