SID-IM
Ion mobility (IM) is a gas-phase separation technique that separates ions based on their size, charge, and shape. In our lab, we first couple surface-induced dissociation (SID) to IM to increase our understanding of protein complex structure and subunit connectivity using Waters Synapts1–3 (SID-IM, IM-SID, or SID-IM-SID). IM can separate any overlapping protein complex precursors and subunit products, which improves pre- and post-SID data interpretation and protein complex characterization. Currently, we are developing this technique on different IM platforms, e.g., Waters Cyclic4 (Figure 1.), Bruker FT-ICR5 (Figure 2.), and Bruker timsTOF Pro. Additionally, with IM, we are able to perform surface-induced unfolding (SIU) experiments to study the protein complex stability and restructuring by applying a range of SID voltages, recording the shift of the arrival time or collision cross section (CCS), and generating the SIU fingerprint (Figure 3.).
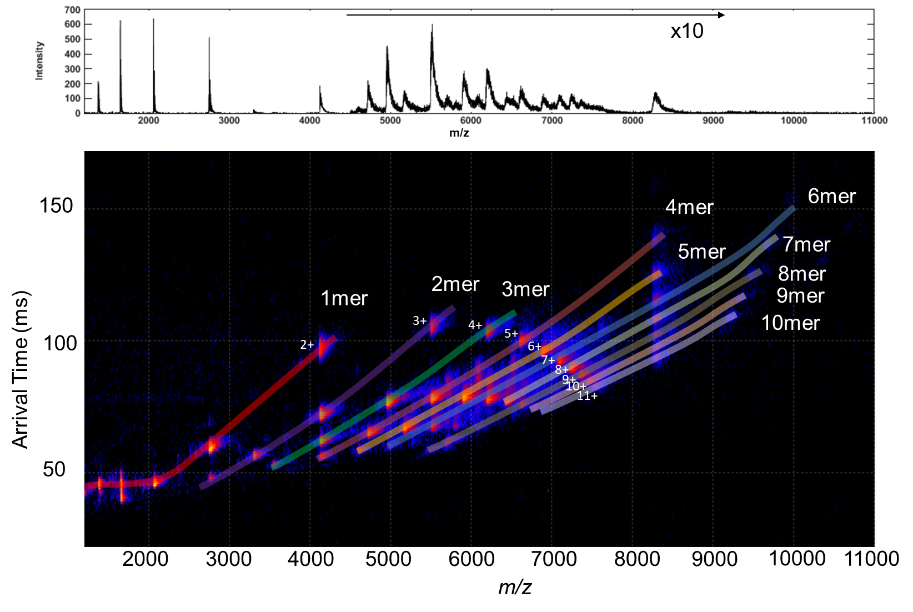
Figure 1. Top) SID spectrum of the 11-mer holoTRAP; Bottom) SID Mobiligram of the 11-mer holoTRAP using Waters Cyclic.
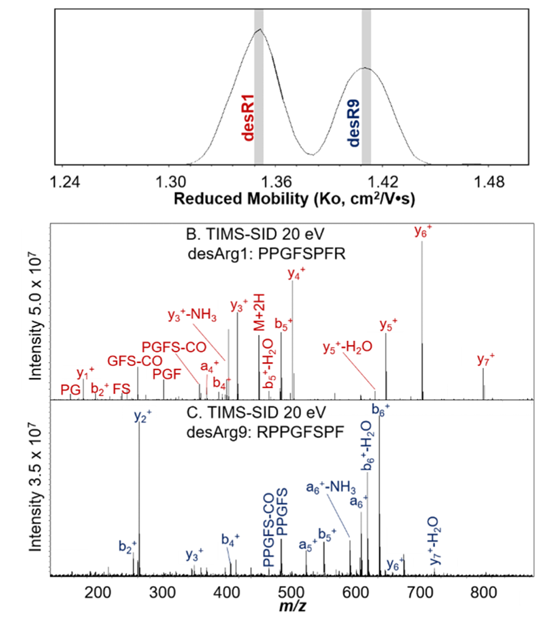
Figure 2. The isomeric desArg1 and desArg9 bradykinins were separated using TIMS based on their mobilities, and the corresponding peptides was assigned based on the SID fragmentation spectra using Bruker FT-ICR.
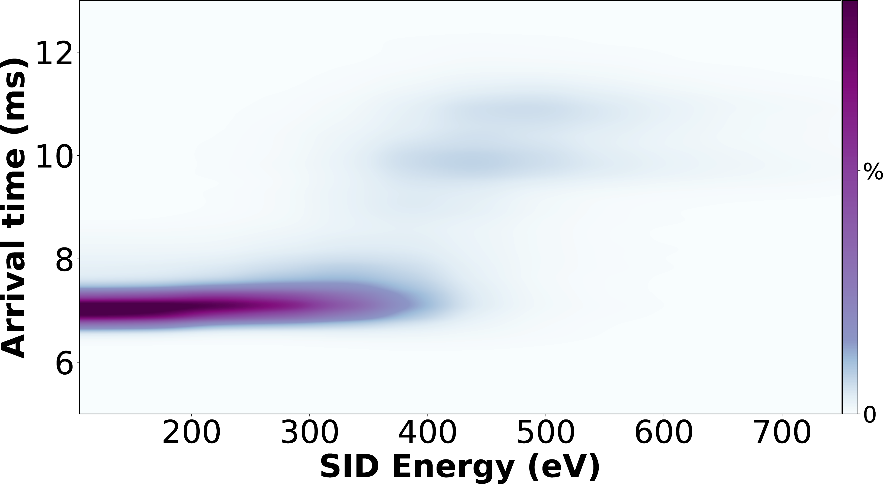
Figure 3. SIU fingerprint of 11+ homotetramer streptavidin using Waters Synapt G2.
1. Song, Y.; Nelp, M. T.; Bandarian, V.; Wysocki, V. H. Refining the Structural Model of a Heterohexameric Protein Complex: Surface Induced Dissociation and Ion Mobility Provide Key Connectivity and Topology Information. ACS Cent. Sci. 2015, 1 (9), 477–487. DOI: 10.1021/acscentsci.5b00251. [Request reprint © (pdf)]
2. Zhou M, Jones CM, Wysocki VH. Dissecting the large noncovalent protein complex GroEL with surface-induced dissociation and ion mobility-mass spectrometry. Anal Chem. 2013 Sep 3;85(17):8262-7. DOI: 10.1021/ac401497c.[Request reprint © (pdf)]
3. Zhou M, Huang C, Wysocki VH. Surface-induced dissociation of ion mobility-separated noncovalent complexes in a quadrupole/time-of-flight mass spectrometer. Anal Chem. 2012 Jul 17;84(14):6016-23. DOI: 10.1021/ac300810u. [Request reprint © (pdf)]
4. Snyder, D. T.; Jones, B. J.; Lin, Y.-F.; Cooper-Shepherd, D. A.; Hewitt, D.; Wildgoose, J.; Brown, J. M.; Langridge, J. I.; Wysocki, V. H. Surface-Induced Dissociation of Protein Complexes on a Cyclic Ion Mobility Spectrometer. Analyst 2021, 146 (22), 6861–6873. DOI: 10.1039/D1AN01407B. [Request reprint © (pdf)]
5. Panczyk, E. M.; Snyder, D. T.; Ridgeway, M. E.; Somogyi, Á.; Park, M. A.; Wysocki, V. H. Surface-Induced Dissociation of Protein Complexes Selected by Trapped Ion Mobility Spectrometry. Anal. Chem. 2021, 93 (13), 5513–5520. DOI: 10.1021/acs.analchem.0c05373. [Request reprint © (pdf)]
ECCR/ECD-SID
In collaboration with eMSion we have developed a combination electron capture dissociation and surface induced dissociation (ECD-SID) device. The device has the ECD portion in the front with a split lens SID device1 at the back and is situated after the quadrupole and before the C-Trap and HCD cell in a Thermo Q Exactive UHMR. This device can be operated either in ECD mode (with or without HCD activation after), SID mode, or ECD-SID mode depending on the goal of the experiment. In addition, the device can be tuned for electron capture charge reduction (ECCR) with or without SID, taking advantage of the fact that electron capture is often observed without dissociation of the protein or complex. This can be beneficial in the study of heterogeneous samples for which charge reduction better resolves the individual species. We are using this device to covalently fragment proteins and their complexes (for ligand mapping and structural studies), to dissociate complexes into their subunits using SID (to learn more about protein structure), and to study heterogeneous samples (such as glycoproteins and nanodiscs).
1. Snyder, D. T.; Panczyk, E. M.; Somogyi, A.; Kaplan, D. A.; Wysocki, V.; Simple and Minimally Invasive SID Devices for Native Mass Spectrometry. Analytical Chemistry 2020,92, 16, 11195–11203. DOI: 10.1021/acs.analchem.0c01657. [Request reprint © (pdf)]
